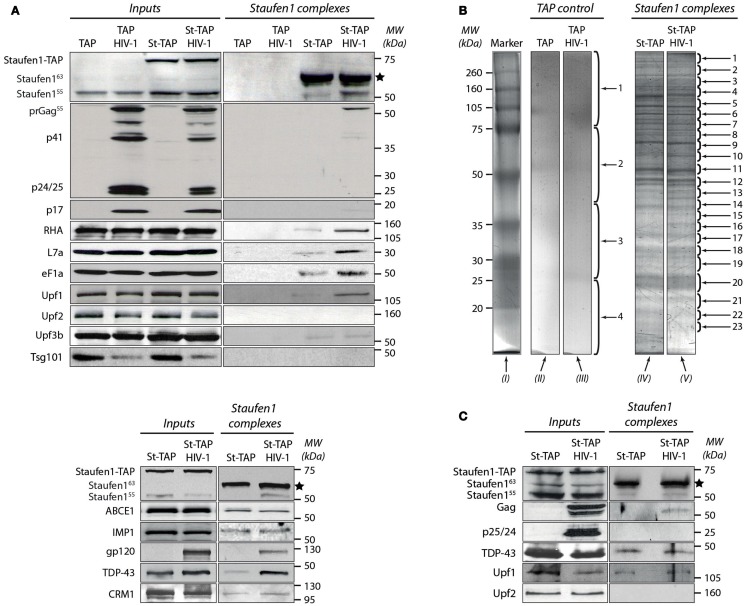
Figure 2.
Characterization of the tandem affinity-isolated Staufen155 kDa RNPs by western blot and mass spectrometry analysis. (A) Separation of proteins from cell lysates (Inputs) before (left panels) and after (right panels) tandem affinity purification (Staufen1 complexes) 0.25 mg of total protein obtained from extracts of TAP (control) or Staufen1-TAP expressing cell lines in the absence or presence of HIV-1 were used for affinity purification. Initially, the membrane was blotted with mouse anti-Staufen1 monoclonal antibody shown on the top left and right panels. The star (*) indicates the position of the purified St-CBP protein. (B) The proteins from the eluted Staufen155 kDa complexes and those from TAP alone (control) were purified from 75 mg of total protein, separated on SDS-PAGE and subsequently stained with Bio-Safe Coomassie Blue. Four bands were excised from gel lanes (II) and (III) for the identification of potential contaminating proteins. 23 bands from St-TAP and St-TAP HIV-1 (lanes IV and V) were excised and analyzed with mass spectrometry. Lane I represents the molecular weights in kDa of the protein standards. (C) Dual affinity purification of Staufen1 complexes derived from Jurkat T St-TAP stable cell lines in the absence or in the presence of HIV-1.