Table 1.
Catalyst screening for enantioselective cyclopropanation of styrene with ethyl α-diazobutanonate: Rh2(S-BPTTL)4= dirhodium(II) tetrakis[N-2,3-naphthaloyl-(S)-tert-leucinate]; Rh2(S-NTTL)4= dirhodium(II) tetrakis[N-1,8-naphthaloyl-(S)-tert-leucinate]; Rh2(S-TBPTTL)4= dirhodium(II) tetrakis[N-tetrabromophthaloyl-(S)-tert-leucinate];
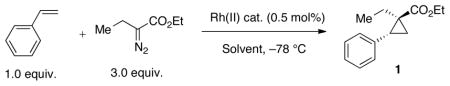 ; ; | ||||
|---|---|---|---|---|
| Rh(II) Catalyst | Solvent | %yda | drb | %eec |
| Rh2(S-PTTL)4 | Hexanes | 95 | 92:8 | 79 |
| Rh2(S-BPTTL)4 | Toluene | 80 | 88:12 | 73 |
| Rh2(S-NTTL)4 | Toluene | 61 | 75:25 | 45 |
| Rh2(S-TBPTTL)4 | CH2Cl2 | 6 | 97:3 | 11 |
| Rh2(S-TBPTTL)4 | Toluene | 10 | 88:12 | 16 |
| Rh2(S-TBPTTL)4 | Hexanes | 23d | 84:16 | 16 |
| Rh2(TPA)(S-PTTL)3 | Toluene | 66 | 95:5 | 81 |
| Rh2(TPA)(S-PTTL)3 | Hexanes | 91 | 96:4 | 88 |
Isolated yields.
Measured by GC.
Measured by chiral HPLC.
Run at −60 °C for 2 hours. No reaction occurred at −78 °C.
