Scheme 2.
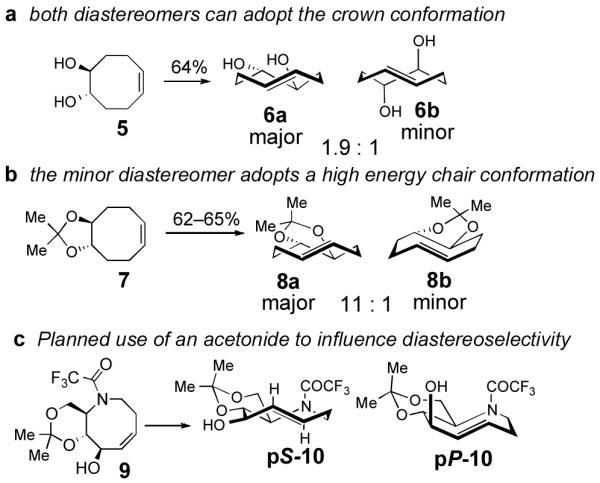
(a) Like most cyclooctenes with stereogenic centers, the photoisomerization of cyclooctene 5 proceeds with poor diastereoselecitvity. (b) The high diastereoselectivity observed in photoisomerization of cyclooctene 7 is attributed to the acetonide ring fusion, which would force the minor diastereomer to adopt a high energy chair conformation. (c) Incorporation of an acetonide in 5-aza-cyclooctene 9 is expected to prefer the formation of pS-10.
