Figure 1.
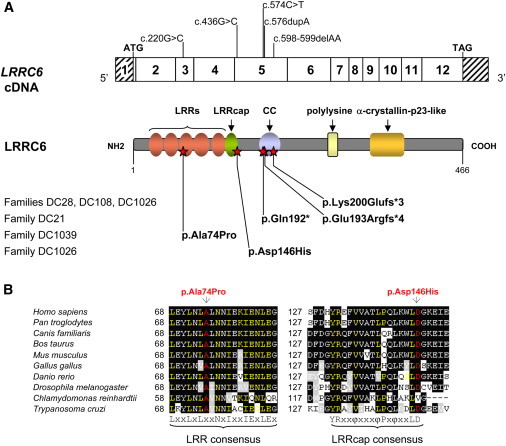
LRRC6 Mutations and Their Impact at the Protein Level in Individuals with PCD
(A) Exonic organization of the human LRRC6 cDNA, in which are shown the mutations (top) and domain-organization model of the corresponding protein (middle). The mutations’ impact at the protein level is shown for the five families described in this study (bottom). The twelve exons are indicated by empty or hashed boxes, depicting translated or untranslated sequences, respectively. According to the LRRC6 domain-organization model, derived from predictions by NCBI and UniProt/Swiss-Prot, the protein contains four LRR domains (amino acids 23–42, 46–65, 68–87, and 90–112), one modified LRR domain (amino acids 115–130) and a subsequent LRRcap (amino acids 131–146), a coiled-coil domain (amino acids 178–204), a polylysine motif (amino acids 272–286), and an α-crystallin-p23-like domain (amino acids 332–381).
(B) A partial protein alignment of LRRC6 shows the evolutionary conservation of the third LRR motif and the LRRcap domain, which contains the two amino acid substitutions identified in this study.
