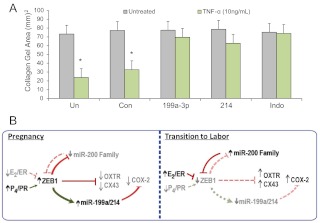
Fig. 5.
miR-199a–3p/miR-214 repression of COX-2 inhibits myometrial contractility to the same extent as indomethacin. A, hTERT-HM human myometrial cells were either untransfected (Un), transfected with a scrambled miRNA mimic (Con), or with mimic for miR-199a–3p or miR-214, embedded in collagen gels and cultured ± TNF-α. A parallel set of untransfected cells embedded in collagen was cultured ± indomethacin, as a positive control for COX-2 inhibition. Contraction of the collagen gels was significantly inhibited by miR-199a–3p and miR-214 transfection after 24 h, when compared with gels containing cells that were either untransfected or transfected with scrambled-control mimics. Data are the mean ± sem of collagen gel area values from three replicate experiments, each conducted in duplicate; *, P < 0.05. B, ZEB1 serves a pivotal role in mediating opposing effects of P4 and E2 on myometrial contractility during pregnancy and labor. Based on the findings presented herein and in our previous work (27), the following mechanism is proposed. During pregnancy, increased circulating levels of P4 and of PR function and decreased levels of E2/ERα activity mediate up-regulation of ZEB1 in myometrium. This causes suppression of the miR-200 family and the CAP genes, OXTR and CX43. The increased ZEB1 also binds to response elements upstream of the miR-199/miR-214 cluster to enhance its expression, resulting in suppression of COX-2 and prostaglandin synthesis. During the transition to labor, the decline in PR function and increase in ERα activity in myometrium result in a down-regulation of ZEB1. This permits up-regulation of the miR-200 family, which further suppresses ZEB1 and inhibits ZEB2. This, in turn, allows de-repression of OXTR and CX43. The decline in ZEB1 also results in decreased expression of the miR-199a/miR-214 cluster, which permits up-regulation of their target, COX-2, and the synthesis of contractile prostaglandins. Collectively these molecular events contribute to the induction of uterine contractility, leading to labor.