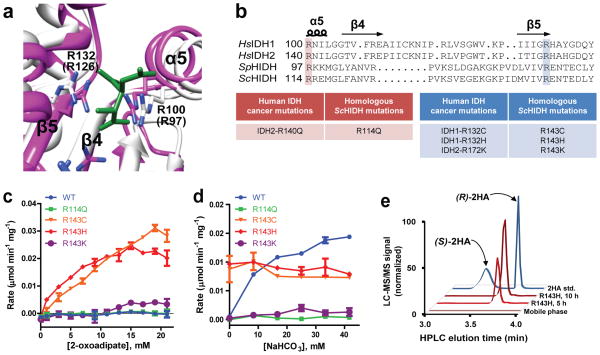
Figure 2. ScHIDH mutants catalyze the NADH-linked production of 2-hydroxyadipate.
(a) Superimposition of the active site for SpHIDH19 (white) onto HsIDH118 (pink; complex with isocitrate in green). Residues Arg100 and Arg132 of HsIDH1 are shown, and corresponding residues for SpHIDH are shown in parentheses. (b) Alignment of HsIDH1, HsIDH2, SpHIDH, and ScHIDH. (c) Initial rate of NADH decrease catalyzed by the indicated ScHIDH mutant at varying concentrations of 2-oxoadipate, and 300 μM NADH. (d) Initial rate of NADH decrease catalyzed by the indicated ScHIDH mutant in the presence of varying concentrations of NaHCO3, as well as 15 mM 2-oxoadipate and 100 μM NADH. (e) LC-MS/MS chromatogram showing (R)-2-hydroxyadipate (Q1/Q3 m/z = 377/161) accumulation in a reaction containing ScHIDH-R143H, 2 mM NADH, 2 mM 2-oxoadipate, and 500 mM HEPES at times indicated. A racemic (R/S)-2-hydroxyadipate standard and mobile phase are shown for reference. Unless otherwise specified, all reactions contained 20 mM MgCl2, 40 ng/μl of the indicated purified enzyme, and 100 mM HEPES, pH 7.3. Data points are mean ± s.d. from n=2 reactions. All plots are representative of three independent experiments.