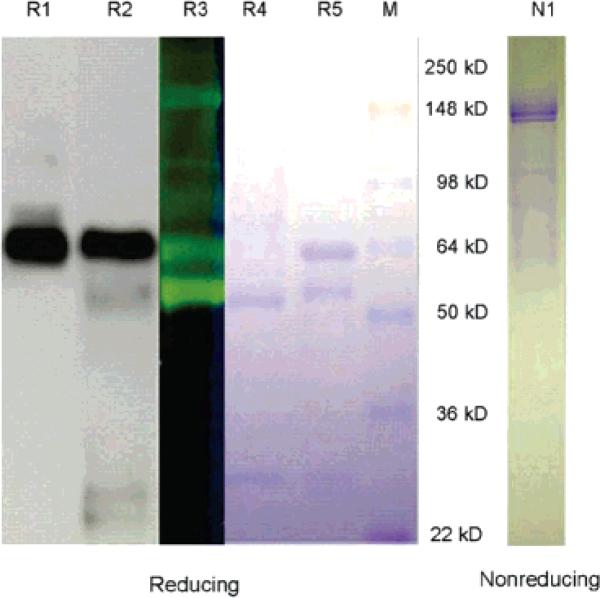
Figure 1.
SDS–PAGE of mAb OX-26 and Fluorescein-Polycefin to demonstrate the conjugation of PMLA with the H-chain of the antibody, and the absence of multiple cross-linking of antibody. Lane N1, Fluorescein-Polycefin. Electrophoresis under nonreducing condition (DTT absent) showing the integral mAb after staining with Coomassie Brilliant Blue. Only the monomeric (150 kDa) but no multiple cross-linked mAbs (>150 kDa) are seen. Lanes R1–R5 and M, electrophoresis under reducing conditions (20 mM DTT) favoring dissociation into H- and L-chains. Detection of mAb OX-26 and its H-chains by Western blotting in lanes R1 and by Coomassie Brilliant Blue staining in lane R5. Detection by Western blotting in lane R2, by Fluorescein-specific fluorescence in lane R3, and by Coomassie Brilliant Blue in R4. Lane M, molecular mass markers. Bands in positions 150–165 kDa (R1–R5) refer to H-chain or a large fragment after a limited proteolytic nicking of the samples. Bands of approximately 25 kDa (R4 and R5) refer to L-chain. Fluorescence of the H-chain (fragment) in lane R3 indicates that this chain is conjugated with the scaffold.