Figure 5. Purification and cell surface binding analysis of recombinant soluble H5-HA (sH5-HA) proteins.
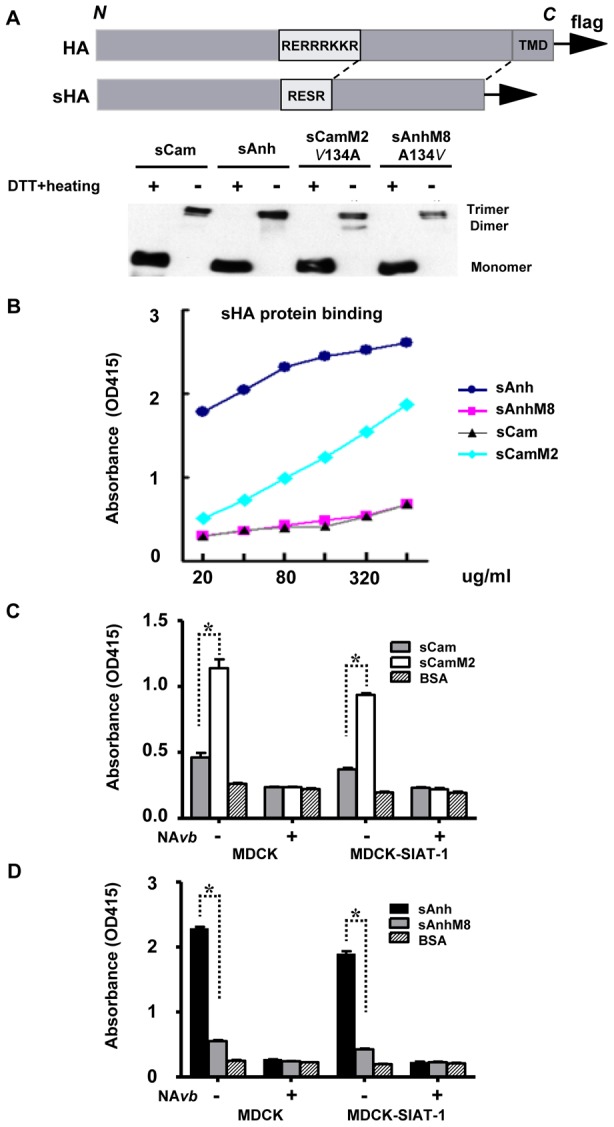
(A) Run on a native gel, purified sH5-HA proteins contain mostly the trimeric form. (B) Dose dependent binding of sH5 HA proteins to MDCK cells. Alanine-134 containing HAs (sAnh and sCamM2) bind strongly to MDCK cells, whereas valine-134 containing HAs (sCam and sAnhM8) bind only weakly. Results are plotted as mean values of two independent experiments. (C) Cell surface binding of sAnh and sAnhM8 proteins to MDCK or MDCK-SIAT-1 cells (more alpha-2,6 linked sialic acid than parental MDCK). Cells were seeded in 96-well plate and grown until confluence with or without NAvb treatment for 2 hrs prior to fixation in 4% paraformaldehyde. Results are shown as means ± SD (n = 3 independent experiments). (D) Cell surface binding of sCam and sCamM2 to MDCK or MDCK-SIAT-1 cells. Cells were grown and treated as in (C). The results are shown as means ± SD (n = 3 independent experiments). Binding of sH5-HA proteins is dependent of sialic acid at cell surface. Similar results were obtained in MDCK and MDCK-SIAT-1 cells. *p<0.01 by the unpaired Student's t-test.
