FIGURE 1.
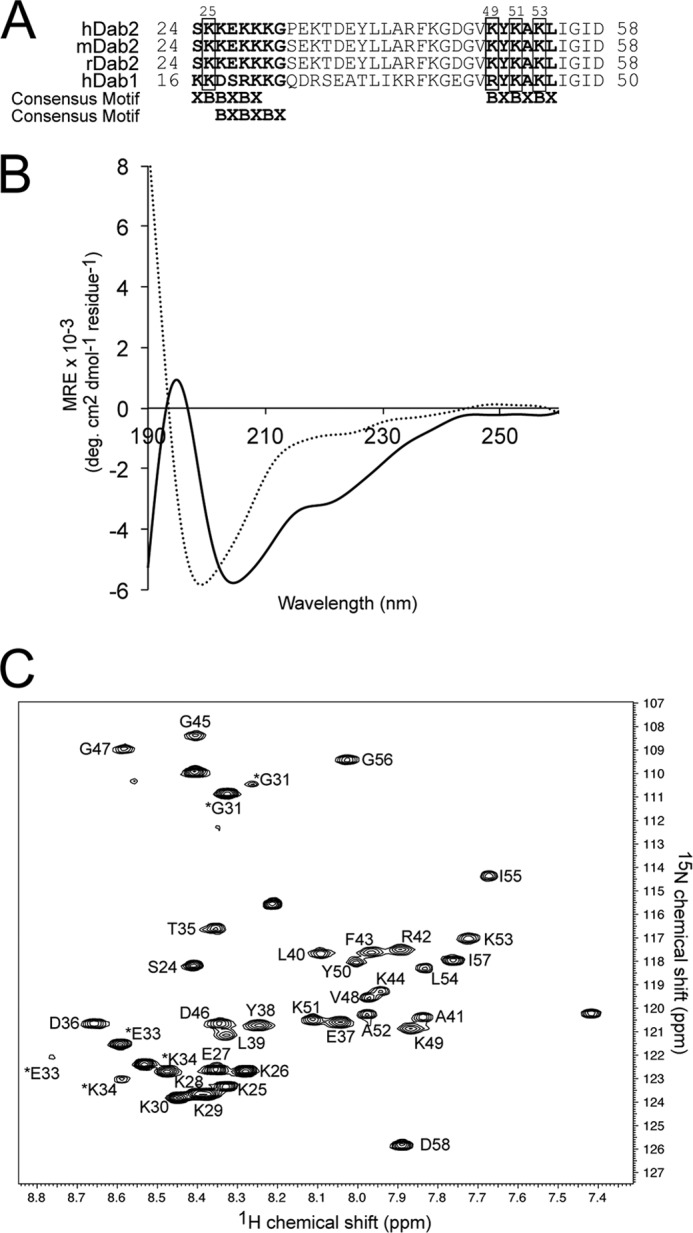
Dab2 SBM is disordered in aqueous solution but becomes structured in the presence of DPC micelles. A, sequences of the closest homologues of Dab proteins corresponding to the SBM region. The two sulfatide-binding sites identified in human Dab2 (hDab2) are bolded, and the key lysine residues are boxed. mDab2, mouse Dab2. B, far-UV CD spectra of Dab2 SBM in the absence (dotted line) and presence (solid line) of DPC micelles. deg, degree. C, two-dimensional 1H, 15N HSQC of uniformly 13C, 15N-labeled Dab2 SBM in DPC micelles. Resonances of the backbone amides are labeled according to human Dab2 protein sequence. Asterisks on Gly-31, Glu-33, and Lys-34 resonances likely are due to cis/trans isomerization of Pro-32. MRE, mean residue ellipticity.
