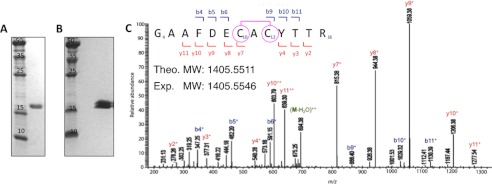
FIGURE 2.
MosR forms a reversible disulfide bond upon oxidation. A, SDS-PAGE gel showing purified reduced MosR. B, double band on nonreducing SDS-PAGE indicates partially oxidized protein. C, LC-MS/MS spectrum of peptide fragment 4GAAFDECACYTTR16 (observed m/z 1405.5546 corresponding to peptide theoretical mass of 1407.5321 Da minus two hydrogen atoms (−2 Da)) obtained after trypsin digestion of oxidized MosR. The fragment ions y7+ and b9+ corresponding to 4GAAFDECAC12 and 10CACYTTR16, respectively (observed m/z 815.38 and 886.40) indicate the presence of disulfide bond between Cys-10 and Cys-12. Fragment ion arising from the neutral loss of water (−18 Da) is marked as M-H2O. Theo. MW, theoretical molecular weight; Exp. MW, experimental molecular weight.