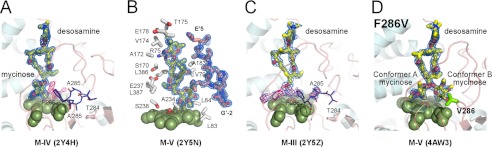
FIGURE 6.
Interactions in the initial recognition complex. M-IV (A), M-V (B and D), and M-III (C) (yellow sticks) are shown fitted to the 2Fo − Fc electron density map (blue mesh) contoured at 1.0 σ. A, superimposition of two alternative conformations for the Thr-284–Phe-286 fragment in the MycG·M-IV complex is shown in dark blue lines. Conformational fluctuations of the Thr-284–Phe-286 fragment correlate with the residual electron density in the Fo − Fc map (pink mesh) contoured at 2.5 σ. B, M-V is shown surrounded by the selected residues (gray sticks) within 5 Å, including the N terminus of the symmetry related molecule (light pink). To enhance clarity, not all amino acid residues are shown. The heme iron axial water ligand is shown as a small red sphere. C, macrolactone and desosamine sugars in M-III are clearly defined in the 2Fo − Fc electron density map (blue mesh) contoured at 0.6 σ, whereas the javose moiety explores the space above the heme macrocycle and thus could not be positioned in the residual Fo − Fc map (pink mesh) contoured at 2.5 σ. D, F286V mutant with M-V bound in two alternative conformations, A and B. Conformation B points toward the hydrophobic cavity gated by residue 286.