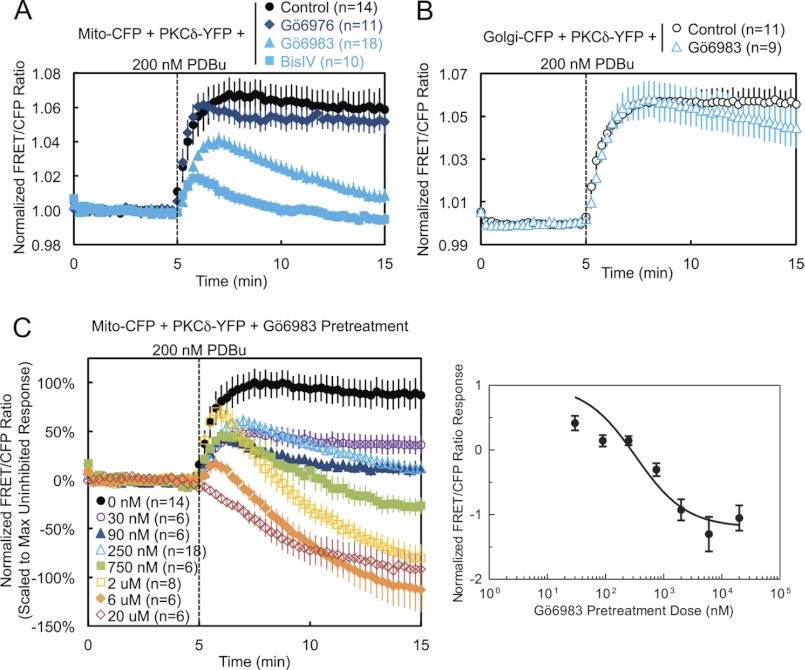
FIGURE 4.
Pharmacological inhibition demonstrates that PKCδ interaction with mitochondria is dependent on novel PKC activity. A, COS-7 cells co-transfected with Mito-CFP and PKCδ-YFP were pretreated with either 500 nm of the conventional PKC inhibitor Gö6976, 250 nm of the general PKC active site inhibitor Gö6983, or 2.5 μm of the general PKC substrate-uncompetitive inhibitor BisIV for at least 20 min at 37 °C and compared with unpretreated cells in their FRET ratio responses to 200 nm PDBu stimulation. B, COS-7 cells co-transfected with Golgi-CFP and PKCδ-YFP were pretreated with 250 nm Gö6983 for at least 20 min at 37 °C and compared with unpretreated cells in their FRET ratio responses to 200 nm PDBu stimulation. C, COS-7 cells co-transfected with Mito-CFP and PKCδ-YFP were pretreated with the indicated concentrations of Gö6983 for at least 20 min at 37 °C and compared with unpretreated cells in their FRET ratio responses to 200 nm PDBu stimulation (left panel). The means ± S.E. of the normalized FRET ratios at 15 min (after 10 min of PDBu stimulation) were plotted as a function of the log of the inhibitor concentration to generate a Gö6983 dose-response curve (right panel).