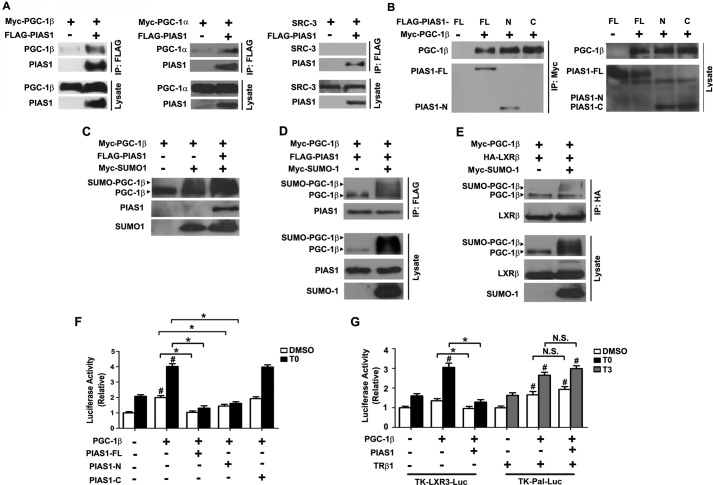
FIGURE 7.
PIAS1 interacts with PGC-1β and affects its SUMOylation and co-activation activity. A, PIAS1 could interact with both PGC-1β and PGC-1α but not with SRC-3. B, N-terminal portion of PIAS1 could associate with PGC-1β. 293T cells were co-transfected for 48 h with the indicated plasmids. Cell lysates were immunoprecipitated with anti-FLAG (A) or anti-Myc (B) antibody. Western immunoblotting was conducted using the tag or SRC-3 antibodies. C, PIAS1 could promote SUMOylation of PGC-1β. 293T cells were co-transfected for 48 h with the indicated plasmids. Cell lysates were prepared in the presence of 20 mm N-ethylmaleimide, and immunoblotting was performed using anti-PGC-1β for the detection of PGC-1β and the tag antibodies for PIAS1 and SUMO1. D and E, effect of PGC-1β sumoylation on its interaction with PIAS1 (D) or LXRβ (E). 293T cells were co-transfected for 48 h with the indicated plasmids. Cell lysates were immunoprecipitated with anti-FLAG (D) or anti-HA (E) antibody. Immunoblotting was conducted using anti-PGC-1β, anti-FLAG, anti-HA, or anti-Myc antibodies. Representative results in A–E are shown from at least two independent experiments. F and G, PIAS1 overexpression blunted the transcriptional co-activation activity of PGC-1β on LXRs but not on TRβ1. HepG2 cells were co-transfected for 48 h with SREBP1c-Pro-Luc (F) or the TK-LXRE3-Luc or TK-Pal-Luc reporter construct (G), together with the indicated plasmids. Cells were subsequently incubated with DMSO, 5 μm T0901317, or 100 nm T3 for 12 h before luciferase activity analysis. Data are presented as means ± S.E., with values normalized to DMSO-treated vector control without PGC-1β (set as 1). *, p < 0.05; #, p < 0.05 by two-way ANOVA. FL, full length. N.S., no significance.