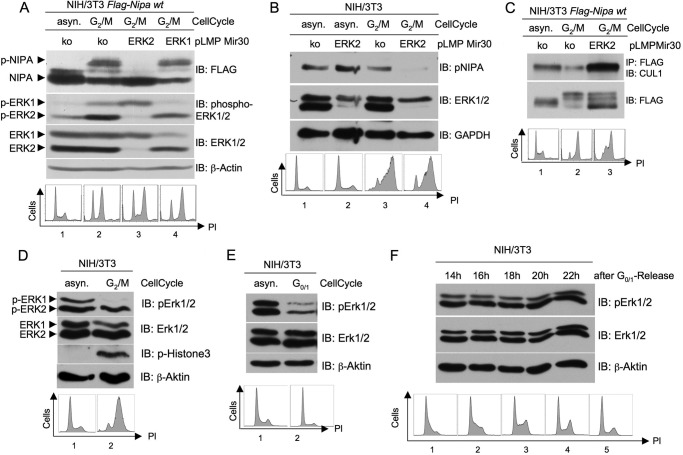
FIGURE 2.
ERK2- but not ERK1-dependent phosphorylation of NIPA at G2/M leads to dissociation of the SCFNIPA complex. A, NIH/3T3 cells stably expressing FLAG-tagged NIPA were retrovirally infected with pLMP vectors encoding a microRNA-30-based shRNA directed against either ERK1 or ERK2 or a control sequence. 48 h after the last infection, cells were synchronized in prometaphase and monitored for NIPA phosphorylation at G2/M. In addition, immunoblot (IB) analyses of phospho-ERK1/2 and ERK1/2 were preformed to guarantee specific knockdown (upper panels), and propidium iodide (PI) cell cycle analyses were performed to ensure correct cell synchronization (lower panels). asyn., asynchronized; ko, knock-out. B, NIH/3T3 cells infected with pLMP vectors encoding either ERK2 or control shRNA were synchronized in prometaphase and monitored for endogenous NIPA phosphorylation at G2/M. Immunoblot analyses of ERK1/2 to guarantee specific knockdown and propidium iodide cell cycle analyses to ensure correct cell synchronization were preformed. C, for co-immunoprecipitation of components of the SCF core complex, NIH/3T3 cells stably expressing FLAG-tagged NIPA were infected with pLMP vectors encoding either ERK2 or control shRNA retrovirus. Thereafter, cells were synchronized at G2/M using a sequential thymidine/nocodazole treatment or left unsynchronized. FLAG-tagged NIPA was immunoprecipitated, and the bound protein fraction was subjected to immunoblot analyses for binding of CUL1. Cell cycle profiles of asynchronous and synchronized cells were analyzed by flow cytometry (lower panel). D, ERK2 is activated at the G2/M transition. NIH/3T3 cells were synchronized at G2/M (D) or G0/G1 (E) and subjected to immunoblot analyses with phospho-ERK and ERK. Phospho-histone 3 immunoblot and propidium iodide cell cycle analyses ensured correct cell synchronization. F, ERK activation during the cell cycle was monitored with immunoblotting of G0/G1-released NIH/3T3 cells. Propidium iodide cell cycle analyses ensured correct cell synchronization.