Background: Escherichia coli O8 and O9a polysaccharide repeat units are synthesized by serotype-specific multidomain (WbdA) mannosyltransferases.
Results: The various WbdA domains are functional when expressed individually.
Conclusion: The number of domains identified in each WbdA protein correlates with the different linkage types formed by the enzyme.
Significance: Modular glycosyltransferases could be exploited for synthesis of precise glycoconjugates with medical and industrial applications.
Keywords: Bacteria, Biosynthesis, Glycoconjugate, Glycosyltransferases, Lipopolysaccharide (LPS), E. coli, O-polysaccharide, Mannosyltransferase, Modular Enzyme, Polymerase
Abstract
The Escherichia coli O9a and O8 polymannose O-polysaccharides (O-PSs) serve as model systems for the biosynthesis of bacterial polysaccharides by ATP-binding cassette transporter-dependent pathways. Both O-PSs contain a conserved primer-adaptor domain at the reducing terminus and a serotype-specific repeat unit domain. The repeat unit domain is polymerized by the serotype-specific WbdA mannosyltransferase. In serotype O9a, WbdA is a bifunctional α-(1→2)-, α-(1→3)-mannosyltransferase, and its counterpart in serotype O8 is trifunctional (α-(1→2), α-(1→3), and β-(1→2)). Little is known about the detailed structures or mechanisms of action of the WbdA polymerases, and here we establish that they are multidomain enzymes. WbdAO9a contains two separable and functionally active domains, whereas WbdAO8 possesses three. In WbdCO9a and WbdBO9a, substitution of the first Glu of the EX7E motif had detrimental effects on the enzyme activity, whereas substitution of the second had no significant effect on activity in vivo. Mutation of the Glu residues in the EX7E motif of the N-terminal WbdAO9a domain resulted in WbdA variants unable to synthesize O-PS. In contrast, mutation of the Glu residues in the motif of the C-terminal WbdAO9a domain generated an enzyme capable of synthesizing an altered O-PS repeat unit consisting of only α-(1→2) linkages. In vitro assays with synthetic acceptors unequivocally confirmed that the N-terminal domain of WbdAO9a possesses α-(1→2)-mannosyltransferase activity. Together, these studies form a framework for detailed structure-function studies on individual domains and a strategy applicable for dissection and analysis of other multidomain glycosyltransferases.
Introduction
Lipopolysaccharides (LPSs) are unique glycolipids that represent major and characteristic components of outer membranes in Gram-negative bacteria. In enteric bacteria, the hydrophobic anchor of LPS, lipid A, is linked via a core oligosaccharide to a hypervariable long-chain repeat unit polysaccharide (1). Variations in the structures of the polysaccharide repeat units define more than 180 different O-antigen serotypes in Escherichia coli (2, 3) and give rise to the term O-polysaccharide (O-PS).5 The lipid A-core oligosaccharide portion of the molecule is synthesized independently of the O-PS, and the pathways converge with a ligation reaction that joins these components at the periplasmic face of the cytoplasmic membrane (1). The completed LPS molecules are then translocated to the outer leaflet of the outer membrane (reviewed in Ref. 4).
The O-PSs of E. coli O9, O9a, and O8 are a family of related structures comprising linear homopolymers of mannopyranose (Manp) (Fig. 1) (3). These glycans are prototypes for O-PSs synthesized via the well distributed ATP-binding cassette transporter-dependent pathway (5, 6). Their synthesis involves biosynthetic intermediates built on the 55-carbon polyisoprenoid lipid acceptor, undecaprenol phosphate. The pathway begins with the formation of und-PP-GlcpNAc by WecA (7–9). WecA is part of the machinery for biosynthesis of the enterobacterial common antigen glycan (10) and initiates the formation of many different E. coli O-PS structures (11). The first dedicated activity in O8/O9/O9a biosynthesis involves transfer of a single α-(1→3)-linked Manp residue by the GDP-Manp-dependent mannosyltransferase, WbdC. Next, WbdB adds two α-(1→3)-linked Manp residues. The WbdCB enzymes are conserved and possess the same activities in all three serotypes (12, 13). Further chain extension builds the repeat unit domain of the O-PSs, and this is achieved by serotype-specific WbdA enzymes (13). The growing chains are terminated by methyl (O8) or phosphomethyl (O9/O9a) groups, which are added by the WbdD proteins. WbdDO8 is a membrane-associated methyltransferase, whereas WbdDO9/O9a is a bifunctional kinase-methyltransferase (14, 15). The terminated glycan is then recognized by a serotype-specific carbohydrate-binding module located at the C terminus of the nucleotide-binding domain component of the ATP-binding cassette transporter, which defines this type of assembly pathway (16). Chain termination is essential for recognition and export (16).
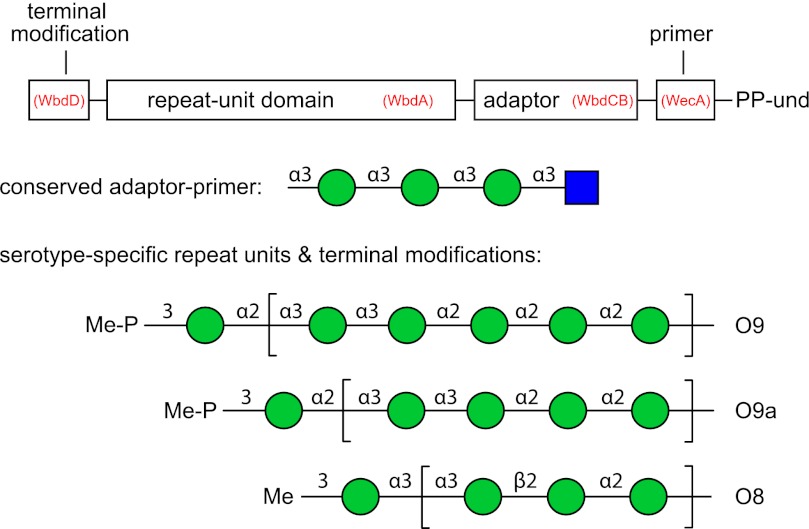
FIGURE 1.
Structures the E. coli O8, O9 and O9a polymannose O-PSs. Each polysaccharide contains four structural regions, the primer, adaptor, repeat unit domain, and terminal modification, which are represented in the schematic in the context of the und-PP-linked biosynthetic intermediate. GlcpNAc is represented by a blue square and Manp by a green circle according to the nomenclature used by the Consortium for Functional Glycomics. The enzymes responsible for the formation of each part of the glycan are identified in parentheses.
The E. coli O9a and O8 O-PSs are identical to the Klebsiella pneumoniae O3 and O5 O-PSs, respectively (17–22), and the genetic loci encoding the corresponding O-PS biosynthesis enzymes are highly conserved (21). Detailed structural studies of the K. pneumoniae O3 and O5 O-PSs reveal conserved reducing termini and terminating residues that cap the variable serotype-specific repeat unit domains. These structural features are consistent with the assigned biochemical activities of the biosynthetic enzymes (Fig. 1) (15, 22, 23).
The NCBI Conserved Domain Database (24) predicts two putative glycosyltransferase domains in WbdAO9a and three in WbdAO8. Each of these domains is predicted to encompass a retaining mannosyltransferase belonging to glycosyltransferase family GT4 in the CAZy Database (25).6 Consistent with these predictions, the WbdA proteins are considerably larger than a typical single-active site enzyme, such as WbdB (43.9 kDa). WbdAO9a and WbdAO8 have predicted sizes of 95.5 and 137 kDa, respectively. Interestingly, the number of glycosyltransferase domains predicted for each of the WbdA homologues is correlated with the number of different linkage types catalyzed by each enzyme. Previous studies proposed that WbdAO9a contained duplicated domains and showed that the domains could be separated, but both were required for O9a biosynthesis (26). Here, we show that WbdAO8 is also a modular enzyme. It is currently unknown whether a specific functional mannosyltransferase activity is associated with each domain. We address this question in WbdAO9a by assessing the activities of proteins with mutated residues in catalytic site motifs that are conserved in GT4 enzymes, and we establish that the purified N-terminal domain of WbdAO9a possesses poly-α-(1→2)-mannosyltransferase activity.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Plasmids, and Growth Conditions
The bacterial strains and plasmids used in this study are described in Table 1. Bacteria were routinely grown in lysogeny broth (LB) medium (27). d-Glucose (0.4%, w/v), d-mannose (0.1%, w/v), l-arabinose (0.2%, w/v), isopropyl β-d-1-thiogalactopyranoside (0.5 mm), kanamycin (50 μg/ml), ampicillin (100 μg/ml), or chloramphenicol (34 μg/ml) was added where appropriate.
TABLE 1.
Bacterial strains and plasmids
| Strain/plasmid | Description or genotype | Reference or source |
|---|---|---|
| Strains | ||
| Top10 | E. coli F−, mcrA, Δ (mrr-hsdRMS-mcrBC), φ80, lacZΔM15, ΔlacX74, deoR, nupG, recA1, araD139, Δ(ara-leu)7697, galU, galK, repsL(Strr), endA1 | Invitrogen |
| CWG634 | E. coli O9a:K−; trp his lac rpsL cpsK30; manA; Smr; Tcr | Ref. 14 |
| CWG636 | E. coli O8:K−; ugd::aacC1 manA; Gmr; Tcr | Ref. 14 |
| CWG901 | E. coli O9a:K− derivative: trp his lac rpsL cpsK30 manA ΔwbdA::aacC1; Smr; Tcr; Gmr | Ref. 64 |
| CWG1009 | E. coli O9a:K− derivative: trp his lac rpsL cpsK30 manA ΔwbdB; Smr; Tcr | Ref. 14 |
| CWG1010 | E. coli O9a:K− derivative: trp his lac rpsL cpsK30 manA ΔwbdC; Smr; Tcr | Ref. 13 |
| CWG1104 | E. coli O8:K− derivative: ugd::aacC1 manA ΔwbdA; Gmr; Tcr | Ref. 13 |
| CWG1105 | E. coli O9a:K− derivative: manA ΔwbdA; Smr; Tcr | Ref. 13 |
| Plasmids | ||
| pBAD24 | Vector containing l-arabinose-inducible promoter; Apr | Ref. 86 |
| pYA3265 | Source of non-polar aphA-3 cassette; Kmr | A. Honeyman via E. Vimr, Ref. 87 |
| pWQ284 | pBAD24 derivative containing a selectable Cm resistance marker; Cmr | Ref. 16 |
| pWQ573 | pWQ284 derivative; NcoI site in cat gene removed; XbaI in MCS replaced by SpeI; Cmr | J. King |
| pWQ575 | pMAL-c2X derivative containing an XmnI/HindIII fragment encoding WbdCO9a; Apr | Ref. 13 |
| pWQ576 | pMALc-2X derivative containing an EcoRI/HindIII fragment encoding WbdBO9a; Apr | Ref. 13 |
| pWQ583 | pWQ575 derivative containing WbdCO9a E275A mutation; Apr | This study |
| pWQ584 | pWQ575 derivative containing WbdCO9a E283A mutation; Apr | This study |
| pWQ585 | pWQ576 derivative containing WbdBO9a E294A mutation; Apr | This study |
| pWQ586 | pWQ576 derivative containing WbdBO9a E302A mutation; Apr | This study |
| pWQ589 | pBAD24 derivative containing a Km resistance cassette; Kmr | B. Clarke |
| pWQ590 | pBAD24 derivative containing an EcoRI/HindIII fragment encoding His10-WbdA1–435O9a; Apr | This study |
| pWQ591 | pWQ284 derivative containing an EcoRI/HindIII fragment encoding His10-WbdA426–840O9a; Cmr | This study |
| pWQ592 | pBAD24 derivative containing an NcoI/SpeI fragment encoding WbdA1–450O8; Apr | This study |
| pWQ593 | pWQ573 derivative containing an NcoI/SpeI fragment encoding WbdA1–450O8; Cmr | This study |
| pWQ594 | pBAD24 derivative containing an NcoI/SpeI fragment encoding WbdA615–1213O8; Apr | This study |
| pWQ595 | pWQ573 derivative containing an NcoI/SpeI fragment encoding WbdA1–865O8; Cmr | This study |
| pWQ596 | pWQ573 derivative containing an NcoI/SpeI fragment encoding WbdA411–1213O8; Cmr | This study |
| pWQ597 | pWQ631 derivative containing WbdAO9a E317A mutation; Kmr | This study |
| pWQ598 | pWQ631 derivative containing WbdAO9a E325A mutation; Kmr | This study |
| pWQ599 | pWQ631 derivative containing WbdAO9a E750A mutation; Kmr | This study |
| pWQ630 | pWQ631 derivative containing WbdAO9a E758A mutation; Kmr | This study |
| pWQ631 | pWQ589 derivative containing WbdD475–708WbdAO9a; Kmr | B. Clarke |
| pWQ632 | pBAD24 derivative containing an EcoRI/HindIII fragment encoding His10-WbdA426–840O9a; Apr | This study |
General DNA Methods
InstaGene Matrix (Bio-Rad) or DNAzol reagent (Invitrogen) was used to purify chromosomal DNA. DNA fragments were PCR-amplified using Pwo DNA polymerase (Roche Applied Science) or PfuUltra High-Fidelity DNA polymerase (Stratagene), using custom oligonucleotide primers (Sigma) containing restriction sites to facilitate cloning. The sequences and features of the oligonucleotide primers are described in supplemental Table S1. The PureLink PCR Purification Kit (Invitrogen) was used to purify DNA fragments from PCRs or restriction digestions, and DNA fragments from agarose gels were purified using the PureLink Quick Gel Extraction Kit (Invitrogen). Plasmid DNA was purified using the PureLink Quick Plasmid Miniprep Kit (Invitrogen). Restriction endonucleases (Invitrogen and New England Biolabs) and T4 DNA ligase (New England Biolabs) were used according to the manufacturer's instructions. DNA sequencing was performed by the Genomics Facility in the Advanced Analysis Centre (University of Guelph).
Bioinformatic Analyses
Multiple sequence alignments were generated using the ClustalW2 server available at the European Bioinformatics Institute Web site (28, 29). Secondary structure predictions were made using the online programs, JPred (30), PROF (31), SCRATCH Protein Predictor (SSPro) (32), and the PSIPRED Protein Structure Prediction Server (33, 34). Secondary structure models were generated based on the consensus of at least three of the secondary structure predictions. Conserved domain predictions were carried out using the NCBI Conserved Domain Database (24, 35, 36). Three-dimensional structural models were created using the Phyre2 server (37).
Generation of Mannosyltransferase Constructs
Segments of the O9a and O8 wbdA genes corresponding to the putative mannosyltransferase domains were PCR-amplified from E. coli CWG634 (O9a) and CWG636 (O8) chromosomal DNA and cloned behind the arabinose-inducible promoter in pBAD24, pWQ284, or pWQ573, using restriction sites introduced by the oligonucleotide primers. To generate pWQ631, wbdAO9a was PCR-amplified from CWG634 and inserted into pWQ589. The PCR-amplified DNA fragment corresponding to the C terminus of WbdD (amino acids 475–708) was then inserted upstream of the wbdA gene. Site-directed mutagenesis of EX7E motifs was carried out using the QuikChange method (Stratagene, La Jolla, CA). Briefly, mutations were introduced into plasmids via PCR using oligonucleotide primers incorporating the desired base changes (supplemental Table S1). The PCR products were digested with DpnI and transformed into E. coli Top10. All constructs were confirmed by restriction endonuclease digestion and/or by sequencing.
Purification of Polyhistidine-tagged WbdAO9a Domains
Cultures (500 ml) of E. coli Top10 containing pWQ590 (encoding N-WbdAO9a) or pWQ632 (C-WbdAO9a) were grown in LB medium at 37 °C until an A600 of ∼0.3 was reached. Cultures were transferred to 20 °C and grown until midexponential phase (A600 = 0.6). Recombinant protein expression was then induced overnight at 20 °C by the addition of 0.2% arabinose. Cells were collected by centrifugation and resuspended in 25 ml of buffer A (20 mm BisTris, pH 7.0, 250 mm NaCl, 0.5% (w/v) glycerol), prior to lysis by sonication with intermittent cooling on ice. Unbroken cells and large debris were removed by centrifugation at 12,000 × g for 20 min, and the resulting cell-free lysate was centrifuged at 100,000 × g for 60 min to separate the membrane and soluble fractions. Protein purifications were performed by fast protein liquid chromatography (FPLC) using an ÄKTA Explorer system (GE Healthcare). Soluble material from the cell-free lysate was loaded onto a 5-ml HiTrap chelating HP column (Amersham Biosciences) charged with nickel ions. The column was washed sequentially with 3 column volumes of buffer A containing 0, 50, and 75 mm imidazole. N-WbdAO9a and C-WbdAO9a were eluted in buffer A containing 125 mm imidazole. The protein-containing fractions were pooled, and the buffer was exchanged with storage buffer (20 mm BisTris, pH 7.0, 50 mm NaCl), using a desalting column (GE Healthcare) according to the manufacturer's instructions. The samples were then concentrated using a 30,000 MWC Vivaspin filtration unit (Sartorius Biolab Products) according to the manufacturer's instructions. The protein concentrations were determined using the A280, based on theoretical extinction coefficients of His10-N-WbdAO9a (42,080 m−1 cm−1) and His10-C-WbdAO9a (81,150 m−1 cm−1) predicted by the ProtParam program (38). The protein concentrations of His10-N-WbdAO9a and His10-C-WbdAO9a were typically 2 mg/ml.
Structural Analysis of the O-PS Produced in Cells Containing Mutations in the C-terminal EX7E Motif of WbdAO9a
The LPS from CWG901 (pWQ599 expressing WbdA E750A) was purified from 18 liters of culture according to the hot aqueous-phenol method described by Westphal and Jann (39). The polysaccharide was released from LPS by mild acid hydrolysis (2% acetic acid, 100 °C, 3 h), isolated by gel filtration chromatography as described elsewhere (22), and analyzed by NMR spectroscopy. Spectra for DQCOSY, TOCSY (mixing time 120 ms), NOESY (400 ms delay), and gHSQC experiments were obtained on a Varian Unity 500 NMR spectrometer in D2O at 25 °C. Standard pulse sequences were applied with reference to internal acetone (1H, 2.23 ppm; 13C, 31.45 ppm). The AQ time was maintained at 0.8 s for H-H correlations and 0.25 s for gHSQC. 256 data series were collected for all spectra.
In Vitro Mannosyltransferase Reactions Using Synthetic Acceptors
Two synthetic fluorescein-tagged acceptors were used as substrates for N-WbdAO9a and C-WbdAO9a. Acceptor A (α-Manp-(1→2)-α-Manp-(1→2)-α-Manp-(1→3)-α-Manp) represents the repeat unit of the O9a antigen. Acceptor B (α-Manp-(1→3)-α-Manp-(1→3)-β-GlcpNAc) represents the conserved reducing terminal trisaccharide of the O8 and O9a antigens. Their synthesis has been described elsewhere (15, 40, 41), and they have been used to characterize the WbdDO9a-mediated chain-terminating activity (15) as well as activities of the full-length WbdAO9a and WbdAO8 mannosyltransferases (13). Standard reactions were performed as described previously (13) in 10-μl reaction volumes of buffer B (50 mm HEPES, 20 mm MgCl2, 1 mm dithiothreitol, pH 7.5) containing 10 μm enzyme (purified N-WbdAO9a, C-WbdAO9a, or both), 0.5 mm acceptor, and 5 mm GDP-Manp. Reactions were incubated for 30 min at 25 °C and terminated by the addition of an equal volume of stop solution (50% (v/v) acetonitrile, 1% (w/v) SDS, 10 mm EDTA). After diluting 1:4 in 50% (v/v) aqueous acetonitrile, 2-μl portions were spotted on AL SIL G thin layer chromatography plates (Whatman). The plates were developed with ethyl acetate/water/1-butanol/acetic acid (5:4:4:2.5), and fluorescent reaction products were detected with a hand-held UV lamp.
Structural Analyses of Reaction Products
To generate sufficient quantities of product for NMR spectroscopic analyses, 250-μl reactions were carried out as described above using Acceptor B. After 30 min, the reactions were diluted in 1 ml of H2O and loaded onto a C18 Sep-Pak cartridge (Waters). The cartridge was washed extensively with water, and the products were eluted in 2 ml of 60% (v/v) aqueous acetonitrile. The eluted products were then concentrated using a SpeedVac concentrator. MALDI-TOF mass spectra of the reaction products were obtained on a Bruker Ultraflextreme MALDI-TOF/TOF in positive ion mode. All NMR spectra were acquired in D2O at 27 °C on an Agilent VNMRS 700-MHz spectrometer equipped with a cryoprobe. The spectra were referenced to an external standard of acetone (2.22 ppm for 1H and 31.07 ppm for 13C at 27 °C). One-dimensional, gCOSY and tROESY, 1H spectra were obtained for the products generated by N-WbdAO9a alone and by N-WbdAO9a and C-WbdAO9a together. Additionally, a 1H-13C gHSQC spectrum was obtained for the products generated by N-WbdAO9a alone. For all of the 1H spectra, the intensity of the residual HOD peak was decreased using a presaturation pulse sequence, irradiating at 4.76 ppm. The spectral window for the one-dimensional 1H spectra was 8446 Hz (from 10.8 to −1.3 ppm), and a Gaussian function was applied interactively to improve the signal/noise ratio. The spectral windows for the gCOSY and tROESY were 6313 Hz (from 9.5 to 0.5 ppm) in both dimensions. The gCOSY was acquired with 674 increments in F1 and 8 transients in F2, and the tROESY was acquired with 600 increments in F1, 16 transients in F2, and a 0.4 s mixing time. The spectral window for the 1H-13C gHSQC was 5605 Hz (from 9.0 to 1.0 ppm) in F2 (1H dimension, 16 transients) and 28.2 kHz (from 160 to 0 ppm) in F1 (13C dimension, 432 increments). The proton signals were decoupled during acquisition, and the 1JC,H value was set to 140 Hz to determine the appropriate delays. For all of the two-dimensional spectra, sine-bell functions were applied interactively to improve signal/noise ratio.
Protein and LPS PAGE
LPS samples were prepared by proteinase K digestion of whole-cell lysates according to the method of Hitchcock and Brown (42). Protein and LPS samples were analyzed by SDS-PAGE in Tris-glycine buffer (43). Protein was visualized using Simply Blue stain (Invitrogen), and LPS was visualized by silver staining (44). Western immunoblots of LPS were prepared by transferring samples to PROTRAN nitrocellulose membranes (PerkinElmer Life Sciences). Samples were probed at a 1:500 dilution with O9a-specific antiserum (14). Alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (Cedar Lane Laboratories) was used at a dilution of 1:3000, and nitro blue terazolium and 5-bromo-4-chloro-3-indolyl phosphate (Roche Applied Science) were used as substrates for detection.
RESULTS
Modular Architecture of WbdA (Poly)Mannosyltransferase Proteins
WbdC, WbdB, and each WbdA domain (from both O8 and O9a serotypes) are classified into the CAZy GT4 family of glycosyltransferases (25). To determine the boundaries for multiple mannosyltransferase domains in the WbdA proteins, an alignment was performed with PimA from mycobacteria. PimA is a single-active site GDP-Manp-dependent mannosyltransferase belonging to GT4 that is involved in the biosynthesis of phosphoinositol mannosides (45) and was chosen because its structure has been solved (46). The alignment revealed generally low sequence similarity between the various domains and PimA (Fig. 2). However, the two domains of WbdAO9a and two of three domains of WbdAO8 possess a conserved region that includes an EX7E motif found in other retaining glycosyltransferases, including GT4 members (47).6 All but one (WsaF) of the 14 GT4 crystal structures, which includes viral, archaeal, prokaryotic, and eukaryotic representatives, show this motif within the active site of the enzyme, where it is known or predicted to be involved in binding of the nucleotide sugar donor (46, 48–57). The absence of this motif from one domain of WbdAO8 makes it a candidate for the inverting glycosyltransferase activity associated with the O8 mannosyltransferase, adding the β-(1→2)-linked Manp residue in the O8 repeat unit (Fig. 1). The EX7E motif is also present in WbdC and WbdB. The predicted secondary structure of each of the putative WbdA domains was compared with the secondary structure of PimA (determined from its crystal structure (46)). All five WbdA domains have predicted secondary structures similar to that of PimA (data not shown). Collectively, these analyses identified potential boundaries for the domains (Fig. 3) as well as a structural basis for generating constructs expressing the various domains singly or in combination.
FIGURE 2.
Multiple-sequence alignment of the mycobacterial PimA α-mannosyltransferase and the O9a and O8 WbdA mannosyltransferase domains. Conserved residues are highlighted in black, and similar residues are highlighted in gray. The Glu residues of the EX7E motifs are highlighted in red. WbdAO9a Arg80 involved in the WbdAO9 C80R O9 → O9a seroconversion is highlighted in cyan. Alignments were performed using the ClustalW2 server (28, 29).
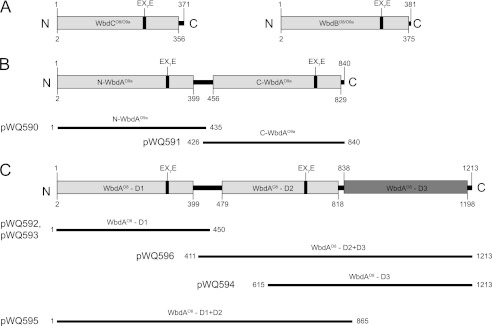
FIGURE 3.
Predicted domains of the O8 and O9a mannosyltransferases. A shows WbdCO8/O9a and WbdBO8/O9a, which both contain a single mannosyltransferase domain. WbdAO9a (B) contains two putative mannosyltransferase domains, and WbdAO8 (C) possesses three. Domain predictions were identified using the NCBI Conserved Domain Database (24). Cloned regions encoding individual domains of the WbdA homologs are identified.
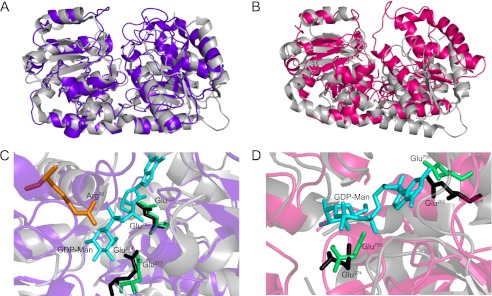
Both WbdAO9a domains show features of a catalytically active glycosyltransferase module (i.e. they possess an EX7E motif and share similar (predicted) secondary structures with PimA). To provide additional insight, three-dimensional models of the N-WbdAO9a and C-WbdAO9a domains were generated using Phyre2 (37). The highest scoring models for N-WbdAO9a (17% sequence identity, 91% sequence coverage, 100% confidence) and C-WbdAO9a (17% sequence identity, 86% sequence coverage, 100% confidence) were based on the structure of sucrose synthase 1, a GT4 enzyme from Arabidopsis thaliana (57). The structure of the mycobacterial PimA protein was overlaid onto the modeled structures of the WbdAO9a domains (Fig. 4). PimA was chosen because it is also a GDP-Manp-dependent mannosyltransferase belonging to the GT4 family, and its structure has been solved in complex with GDP-Manp (46), which may highlight possible binding interactions for the same sugar nucleotide donor in the WbdAO9a domains. The predicted structure of N-WbdAO9a and C-WbdAO9a closely matches PimA (Fig. 4). The typical GT-B fold (i.e. two loosely associated α/β/α Rossmann-like domains, separated by a linker that forms the active site cleft) is clearly evident. The Glu residues of the EX7E motifs fall within the putative active sites of N-WbdAO9a and C-WbdAO9a. In N-WbdAO9a, the Glu residues are almost superimposable on the equivalent Glu residues of the EX7E motif in PimA (Fig. 4, B and D), and those of C-WbdAO9a also occupy a similar position. Like PimA, the carboxylate groups of the first Glu in the EX7E motif of the WbdAO9a domains are predicted to hydrogen-bond with the Manp portion of GDP-Manp. In addition, the carboxylate groups of the second Glu are in a position to hydrogen-bond with the ribose moiety of GDP-Manp.
FIGURE 4.
Structural models of N-WbdAO9a and C-WbdAO9a compared with the mycobacterial PimA mannosyltransferase. A and B show an overlay of the N-WbdAO9a model (purple) and C-WbdAO9a model (magenta) with PimA (gray), respectively. C and D show expanded views of the corresponding modeled active sites. Glu residues of the WbdA EX7E motifs are colored green. Arg80 is involved in the WbdO9 C80R O9 → O9a seroconversion (76) and is highlighted in orange in the N-WbdAO9a model. The Glu residues of the EX7E motif in PimA are in black, and GDP-Manp is shown in cyan. The first Glu residue of the EX7E motif is in a position to hydrogen-bond with the Manp moiety of GDP-Manp. The second Glu residue of the motif is in a position to hydrogen-bond with the ribose moiety of GDP-Manp.
WbdA Mannosyltransferase Domains Are Functional in Vivo When Co-expressed as Separate Domains
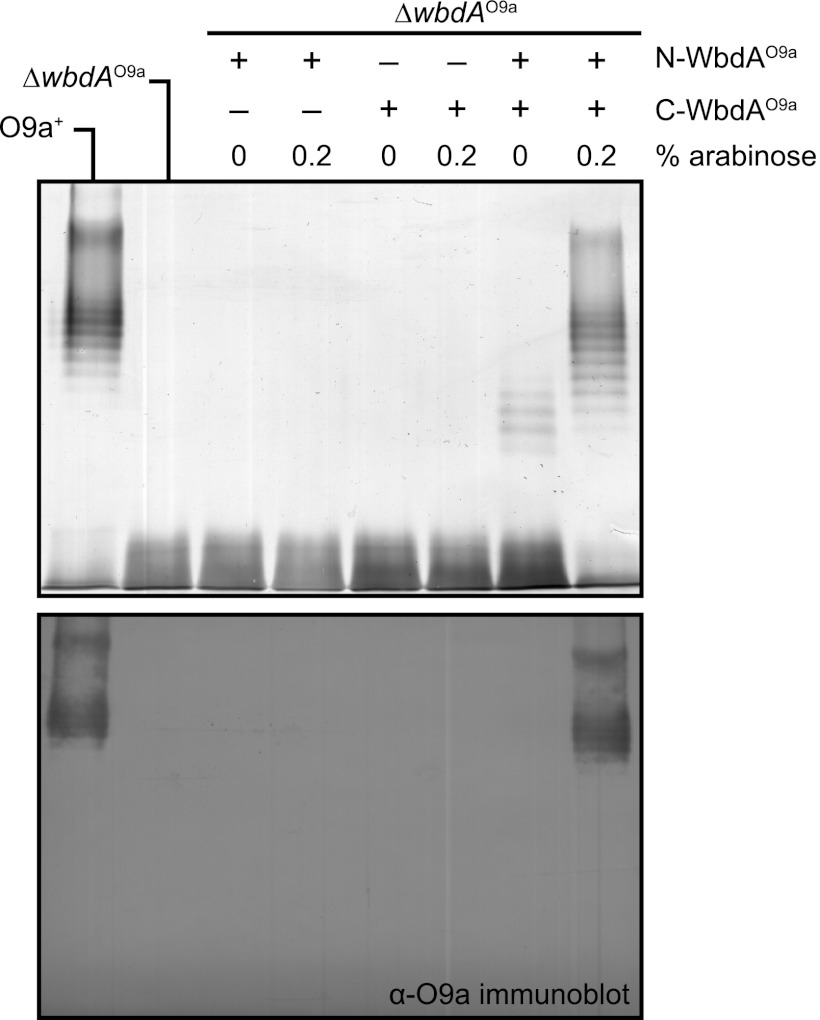
Previous research indicates that the two domains of WbdAO9a could be expressed as independent polypeptides and rescue O9a biosynthesis in a wbdA::Tn1000 mutant (26). However, interpretation of this earlier finding is complicated by the use of a transposon mutant background where wbdA was not completely deleted, and the extent of expression of residual parts of wbdA was not examined. This is a particular concern for a predicted multidomain protein. To unequivocally establish that two functional domains exist in the WbdAO9a protein, constructs were made with the same breakpoints in wbdAO9a used in the earlier study (26), leading to the expression of N-WbdAO9a and C-WbdAO9a (Fig. 3). From secondary structure predictions, the breakpoints fall within predicted α-helices in a region linking the two putative glycosyltransferases domains (data not shown). Neither domain could restore O-PS production when expressed alone in a clean deletion of wbdAO9a (Fig. 5). However, when both domains were expressed as separate polypeptides, O9a O-PS biosynthesis was restored. To generate LPS products of the native size range, 0.2% l-arabinose addition was required for induction of the pBAD promoter. Therefore, as predicted, WbdAO9acontains two separable domains, both of which are required to polymerize the O9a O-PS. In the earlier study, expression of N-WbdAO9a alone in the wbdA::Tn1000 mutant was sufficient to generate a new polymannose glycan, whose structure differed from the O9a antigen (26). This observation could not be replicated in the ΔwbdAO9a background used here, and the disparity in the systems is addressed below.
FIGURE 5.
WbdAO9a contains two separable domains. The results show mutant complementation experiments with CWG1105 (ΔwbdAO9a) expressing N-WbdAO9a (pWQ590), C-WbdAO9a (pWQ591), or N-WbdAO9a and C-WbdAO9a combined (pWQ590 + pWQ591). Top, silver-stained SDS-polyacrylamide gel of LPS samples from whole-cell lysates; bottom, the corresponding Western immunoblot using O9a-specific antiserum. Native O-PS biosynthesis was restored only when both WbdAO9a domains were present.
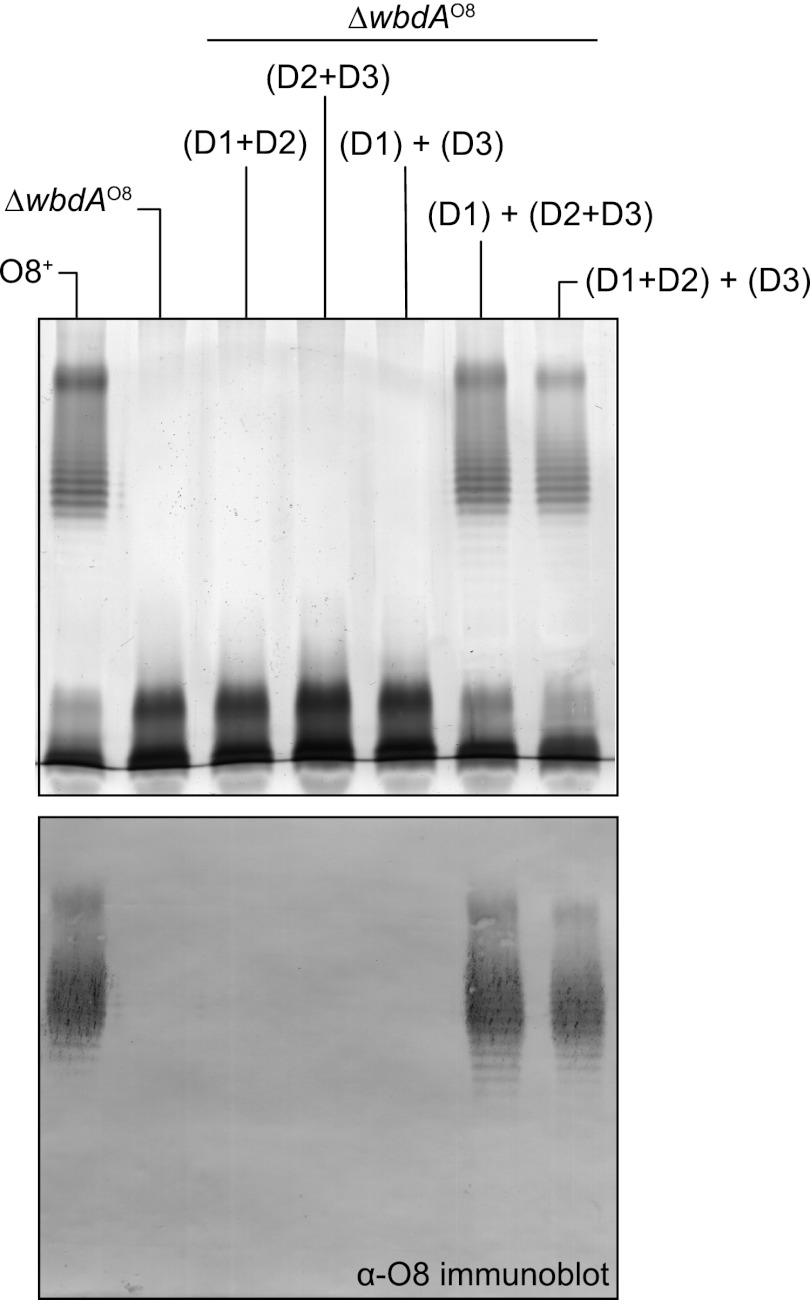
A similar strategy was used to examine the domain architecture of WbdAO8. The breakpoint between WbdAO8-D1 and D2 (Fig. 3C) was based on the predicted domain boundaries and the predicted secondary structures in the regions linking the relevant domains (data not shown). This yielded functional constructs (Fig. 6). In contrast, the logical breakpoint identified between D2 and D3 by secondary structure prediction did not result in a functional isolated D3 domain. Several non-functional constructs were examined with nested breakpoints (data not shown). Ultimately, a conservative construct, ending partway into the adjacent D2 domain, proved to be functional. In the absence of any one of the domains, O-PS biosynthesis could not be restored in the ΔwbdAO8 mutant (Fig. 6). However, O8 synthesis was rescued whenever all three domains were present, regardless of the combination. Thus, like WbdAO9a, WbdAO8 appears to contain separable active domains, all of which are essential for serotype-specific O-PS biosynthesis.
FIGURE 6.
WbdAO8 contains three separable domains. The results show mutant complementation experiments with CWG1104 (ΔwbdAO8) expressing WbdAO8-D1+D2 (pWQ595), WbdAO8-D2+D3 (pWQ596), WbdAO8-D1+D3 (pWQ593 + pWQ594), or WbdAO8-D1+D2+D3 (pWQ592 + pWQ596 or pWQ594 + pWQ595). Top, silver-stained SDS-polyacrylamide gel of LPS samples from whole-cell lysates; bottom, the corresponding Western immunoblot using O8-specific antiserum. Native O-PS biosynthesis was restored only when all three WbdAO8 domains were present.
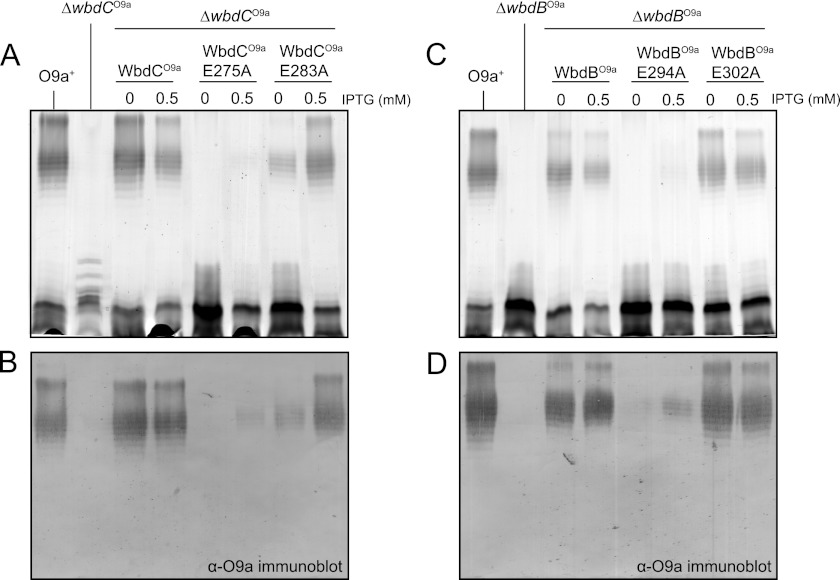
The First Glu Residue of the EX7E Motif Is Crucial for the Activity of WbdCO9a and WbdBO9a
Mutagenesis studies involving several members of the GT4 family confirm the importance of the EX7E motif in enzyme activity, but there is contradictory literature concerning the relative importance of each Glu residue (58–62). In the majority of cases, the first Glu appears to be more important for enzyme activity. Site-directed mutagenesis was used to investigate the importance of the EX7E Glu residues in mannosyltransferases from the O8 and O9a systems, focusing initially on the single-active site enzymes, WbdC and WbdB. Four mutant constructs were generated: MalE-WbdCO9a E275A, MalE-WbdCO9a E283A, MalE-WbdBO9a E294A, and MalE-WbdBO9a E302A. Their activities were confirmed in vivo by assessing their ability to complement the corresponding ΔwbdC and ΔwbdB mutants (Fig. 7). In both cases, complementation was evident without induction, reflecting a leaky promoter (63). The amounts of O-PS-substituted LPS decreased with higher levels of expression following isopropyl β-d-1-thiogalactopyranoside induction. This has been observed previously with wild type genes (13), but although the reasons are unknown, this phenomenon does not affect interpretation of the complementation data. The MalE-WbdCO9a E275A and MalE-WbdBO9a E294A mutants showed a drastic reduction in activity; only trace amounts of O-PS were produced in the presence of isopropyl β-d-1-thiogalactopyranoside inducer, and detection was only possible in the Western immunoblots. In contrast, when MalE-WbdCO9a E283A and MalE-WbdBO9a E302A were introduced into the corresponding deletion mutants, the wild type LPS profiles were restored, and detectable amounts of the O9a antigen were evident even in the absence of inducer. In all complementation experiments, the levels of protein expression were comparable with those observed when the deletion strains were transformed by constructs encoding the wild type genes (data not shown), indicating that the results were not influenced by poor expression or protein degradation. CD spectra of the MalE-WbdCO9a E275A and MalE-WbdBO9a E294A proteins were comparable with those of the wild type enzymes (data not shown), ruling out any problems with folding. These results suggest that the first Glu residue of the EX7E motif plays an important role in the function of the WbdC and WbdB enzymes. In contrast, the second Glu is not essential for activity and O-PS synthesis in vivo, at least under the experimental conditions employed here.
FIGURE 7.
Mutagenesis of Glu residues in the EX7E motifs of WbdCO9a and WbdBO9a. The results show mutant complementation experiments with CWG1010 (ΔwbdCO9a) expressing WbdCO9a (pWQ575), WbdCO9a E275A (pWQ583), or WbdCO9a E283A (pWQ584) (A and B) and CWG1009 (ΔwbdBO9a) expressing WbdBO9a (pWQ576), WbdBO9a E294A (pWQ583), or WbdBO9a E302A (pWQ584) (C and D). Top panels, silver-stained SDS-polyacrylamide gel of LPS samples from whole-cell lysates; bottom panels, the corresponding Western immunoblots using O9a-specific antiserum. O-PS biosynthesis was severely impaired when only the first Glu residues of the EX7E motif were substituted in WbdCO9a and WbdBO9a. IPTG, isopropyl β-d-1-thiogalactopyranoside.
Differential Effects of Mutations in the EX7E Motifs in WbdAO9a
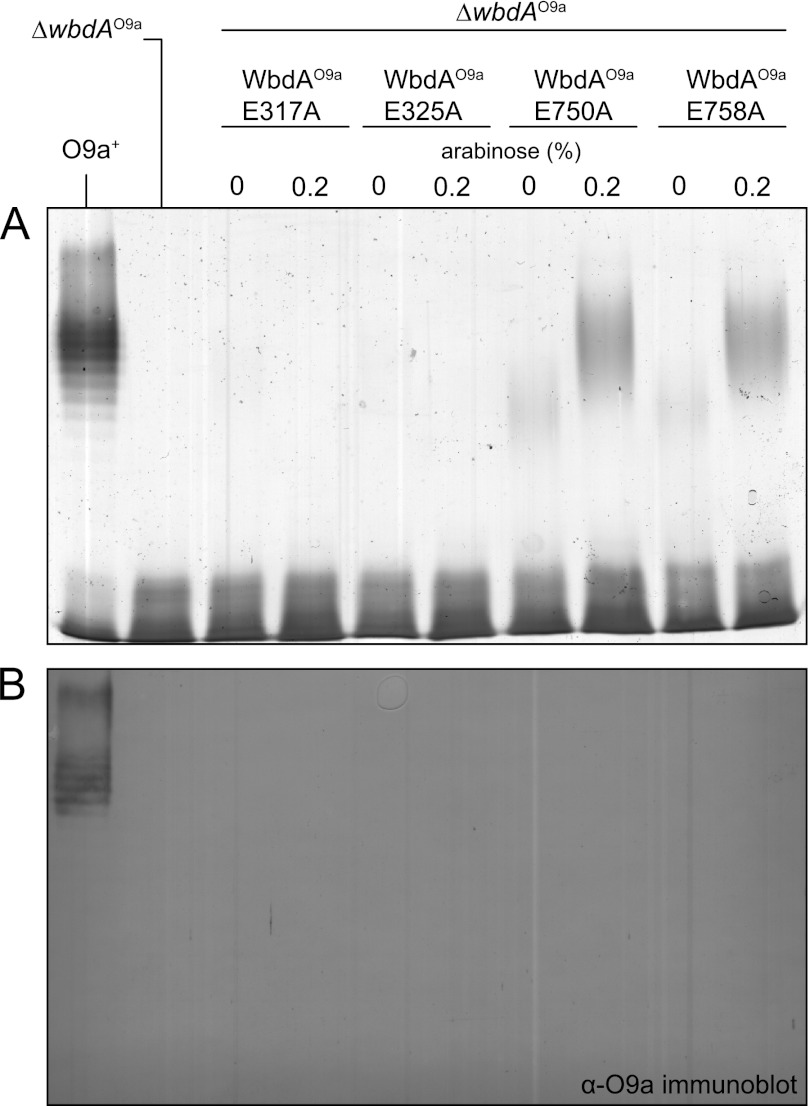
To investigate the importance of the Glu residues in the EX7E motif of the N-WbdAO9a domain, the mutant derivatives WbdD*AO9a E317A and WbdD*AO9a E325A were generated. The constructs used in these experiments contained the C-terminal portion of WbdDO9a (D*, representing residues 475–708), which is responsible for targeting WbdAO9a to the membrane (64). Co-expression of this fragment of WbdD was intended to maximize the potential association of the WbdA domain(s) with the membrane for optimal O-PS synthesis. The approach was successful. O-PS production by WbdD*AO9a was greater than when compared with the level of O-PS produced by the corresponding constructs lacking the C-terminal region of WbdD (data not shown).
The E317A and E325A mutant forms of WbdAO9a were unable to support biosynthesis of O-PS when expressed in ΔwbdAO9a (Fig. 8), indicating that both Glu residues of the N-WbdAO9a EX7E motif are essential for the activity of the enzyme. In contrast, O-PS was produced (evident in high molecular weight LPS in silver-stained PAGE) when mutants in the C-terminal EX7E motif, WbdD*AO9a E750A and WbdD*AO9a E758A, were assessed in ΔwbdAO9a (Fig. 8). However, these molecules lacked the typical “ladder-like” appearance of the native O9a LPS, and they were not detected in Western immunoblots using antibodies specific for the O9a O-PS (Fig. 8B), indicating altered structure and antigenicity.
FIGURE 8.
Differential effects of mutations in the EX7E motifs of WbdAO9a. The results show gene complementation experiments of CWG1105 (ΔwbdAO9a) expressing WbdAO9a E317A (pWQ597), WbdAO9a E325A (pWQ598), WbdAO9a E750A (pWQ599), or WbdAO9a E758A (pWQ630). A, silver-stained SDS-polyacrylamide gel of LPS samples from whole-cell lysates; B, the corresponding Western immunoblot using O9a-specific antiserum. The E317A and E325A mutants were unable to support biosynthesis of O-PS, whereas the E750A and E758A mutant forms resulted in the production of O-PS with an altered structure and antigenicity.
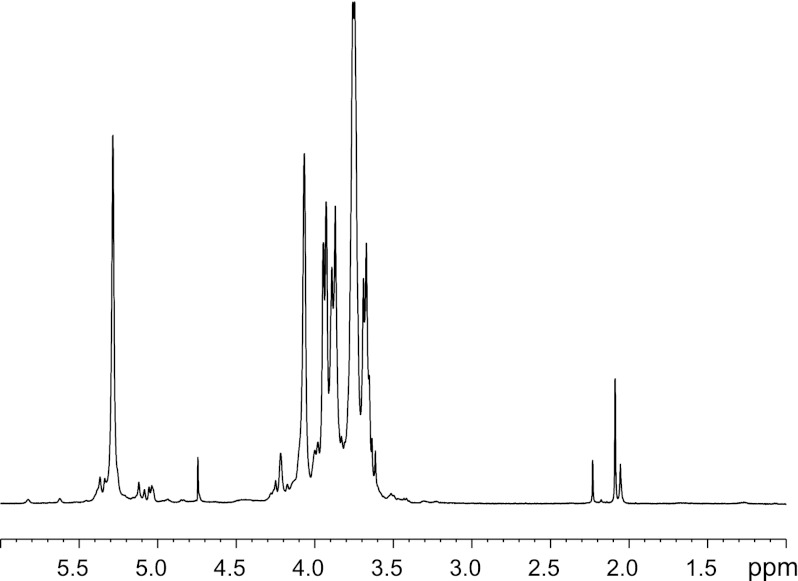
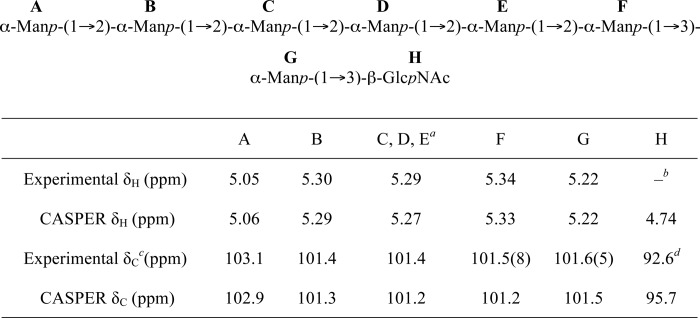
To determine the structure of the novel O-PS resulting from defects in the C-terminal EX7E motif, the LPS from ΔwbdAO9a expressing WbdAO9a E750A was purified and analyzed by NMR spectroscopy. The polysaccharide consisted of a single major monosaccharide, which was identified as α-Manp, based on its characteristic TOCSY pattern (data not shown). The major signals from the repeat unit domain of the glycan were indicative of a Manp homopolymer containing only α-(1→2) linkages (Fig. 9). The α-(1→2) linkages could not be definitively proven by NOE because intraring H-1:H-2 NOEs are always present; therefore, the substitution position was deduced from the low field chemical shift of C-2. The proposed structure is consistent with the prediction of the CASPER program (65, 66) (Table 2). This software predicts 1H and 13C chemical shifts from a database built from mono-, di-, and trisaccharides. This structure is consistent with the observed “smearing” pattern on the silver-stained SDS-polyacrylamide gel (Fig. 8). The typical banding pattern is generated by a heterogeneous mixture of LPS molecules on the surface of the cell that differ in length by one repeat unit in the O-PS. The lack of resolution of individual O-PS-substituted molecules is anticipated when the size increment is a single monosaccharide.
FIGURE 9.
CWG901 (ΔwbdAO9a) cells expressing WbdAO9a E750A produce a (poly-)α-(1→2)-Manp O-PS. Shown is the 1H NMR spectrum for purified LPS substituted with O-PS generated by E. coli CWG901 transformed with pWQ599.
TABLE 2.
Comparison of the chemical shifts for the anomeric protons and carbons of the product generated by N-WbdAO9a using Acceptor B with those predicted by the CASPER database for the octasaccharide shown
a The signals for 1C, 1D, and 1E all overlap and are reported together. There are no differences in the CASPER prediction.
b The HOD peak at 4.76 ppm is too large to see the anomeric signal for β-d-GlcpNAc in the one-dimensional 1H spectrum.
c The experimental values for the 13C chemical shifts were determined from the 1H-13C gHSQC spectrum (supplemental Table S1).
d Weak signal in the 1H-13C gHSQC spectrum.
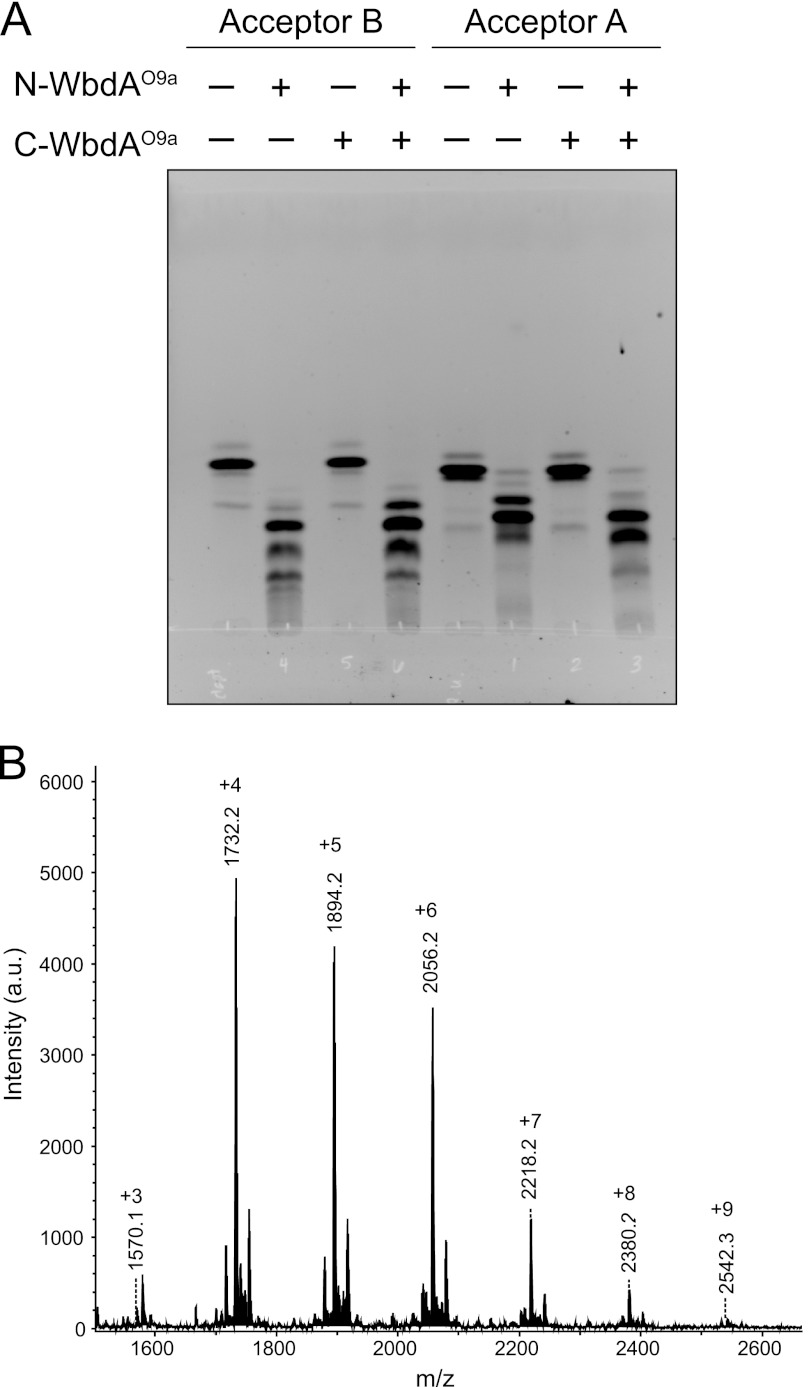
N-WbdAO9a Synthesizes a Poly-α-(1→2)-Manp Glycan Using Synthetic Acceptor Analogues
To investigate the potential mannosyltransferase activity of each WbdAO9a domain, the individual N-terminal polyhistidine-tagged WbdAO9a domains were purified, and their ability to transfer Manp residues from GDP-Manp to the synthetic Acceptors A and B was examined. Under the same reaction conditions, full-length WbdAO9a adds multiple Manp residues to these acceptors to generate a glycan containing the authentic O9a repeat unit (13). N-WbdAO9a was able to transfer multiple Manp residues to both acceptors (Fig. 10A). MALDI MS of the products formed with Acceptor B revealed the addition of up to nine Manp residues (Fig. 10B). The smallest species (m/z 1570.1) corresponds to the sodium adduct of the acceptor plus three additional Manp residues, whereas the largest (m/z 2542.3) is consistent with nine added Manp residues. The major peaks were separated by a mass difference corresponding to one Manp residue (162.1). Unmodified acceptor (m/z 1061) was not evident in the spectrum, indicating that all of the starting material was converted into product.
FIGURE 10.
Analysis of the in vitro products generated by N-WbdAO9a using synthetic acceptors. The reaction products were separated by thin layer chromatography (A), using the fluorescein tag on the acceptors for detection. Note that C-WbdAO9a did not modify either acceptor; nor did its inclusion change the product profile obtained with N-WbdAO9a. The products generated by N-WbdAO9a with Acceptor B were examined by MALDI MS (B), revealing a series of incrementally sized products that differ by the addition of one Manp residue. a.u., arbitrary units.
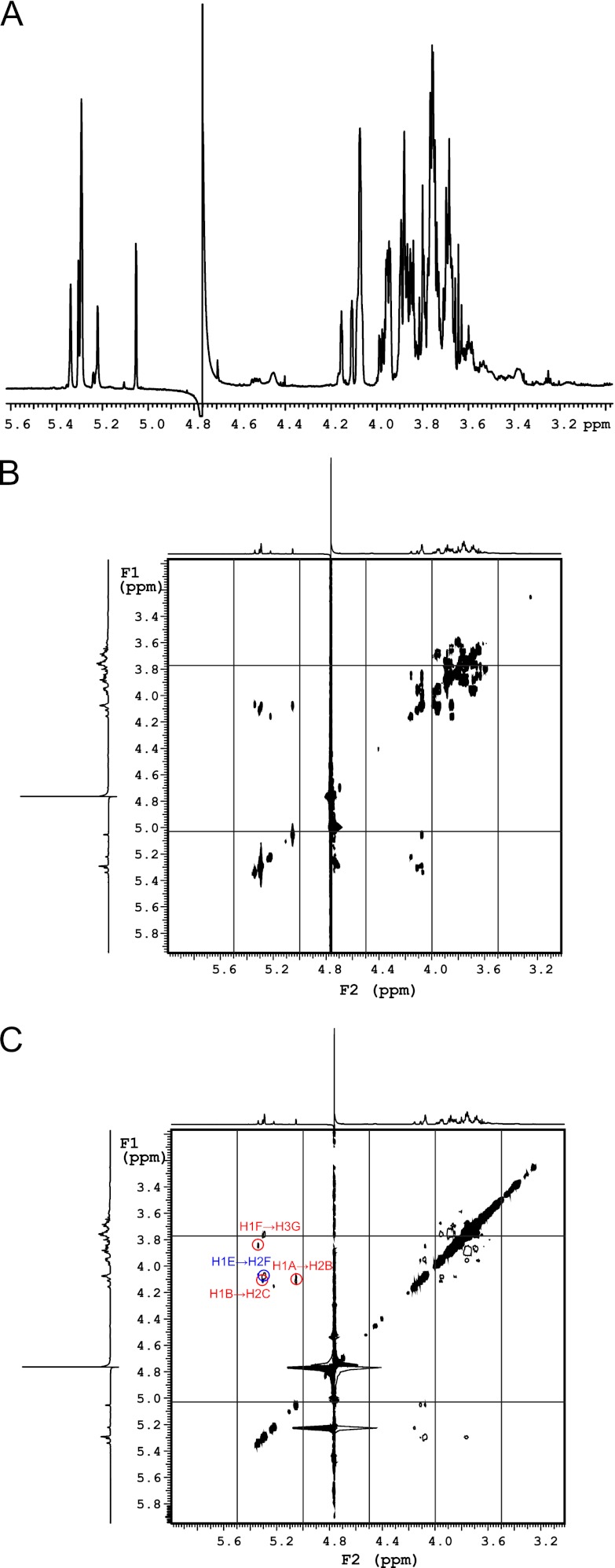
To determine whether a single mannosyltransferase activity or both α-(1→2)- and α-(1→3)-mannosyltransferase activities belong to N-WbdAO9a, NMR spectroscopy was used to determine the structure of the glycan product generated by N-WbdAO9a using Acceptor B. The one-dimensional 1H spectrum revealed five anomeric resonances that integrate in a 1:1:3:1:1 ratio (Fig. 11A). By comparing with the CASPER database (65, 66), we see that the chemical shifts for the anomeric protons and carbons for the major product closely match those predicted for an octasaccharide with the following structure: α-Manp-(1→2)-α-Manp-(1→2)-α-Manp-(1→2)-α-Manp-(1→2)-α-Manp-(1→2)-α-Manp-(1→3)-α-Manp-(1→3)-β-GlcpNAc (Table 3). This structure reflects the trisaccharide acceptor, extended by the addition of five α-(1→2)-linked Manp residues. gCOSY and tROESY experiments were performed and confirmed the identity of the product (Fig. 11). The resonances for H2 and H3 for each of the residues could be correlated with each anomeric signal from the gCOSY spectrum. This information was used to interpret the tROESY spectrum and assign the linkages. Key correlations from the tROESY are labeled in Fig. 11C. The anomeric signal at 5.34 ppm correlated with a signal at 3.84 ppm, which was assigned as the H3 proton in the same residue as the signal at 5.22 ppm. This correlation is indicative of a (1→3)-linked moiety. The anomeric signal at 5.30 ppm had an NOE correlation to a resonance at 4.08 ppm. This signal was assigned to H2 on one of the residues with the anomeric proton at 5.29 ppm, and the correlation is consistent with a (1→2)-linked residue. The anomeric signal at 5.29 ppm correlated with a signal at 4.07 ppm, which is proton H2 on the same ring as the signal at 5.34 ppm. This NOE correlation indicates another (1→2) linkage. The final key correlation is seen between the anomeric proton at 5.05 ppm and a resonance at 4.11 ppm. This signal was assigned to H2 on the same residue as the anomeric proton at 5.30 ppm and is also consistent with a (1→2) linkage. Together, these data demonstrate that N-WbdAO9a possesses poly-α-(1→2)-mannosyltransferase activity and generates the same α-(1→2)-linked polymannan seen in vivo with the full-length WbdAO9a derivative mutated in the C-terminal EX7E motif.
FIGURE 11.
N-WbdAO9a transfers Manp residues in α-(1→2) linkages to Acceptor B. A, 1H NMR spectrum; B, gCOSY spectrum; C, tROESY spectrum.
TABLE 3.
Comparison of the chemical shifts for the protons and carbons of the component Manp in the α-(1→2)-linked polymannose O-PS produced by CWG901 (ΔwbdAO9a) cells expressing WbdAO9a E750A with those predicted by the CASPER database
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| Experimental δH (ppm) | 5.28 | 4.07 | 3.93 | 3.67 | 3.75 | 3.74, 3.88 |
| CASPER δH (ppm) | 5.27 | 4.10 | 3.95 | 3.70 | 3.73 | 3.72, 3.88 |
| Experimental δC (ppm) | 101.4 | 79.6 | 70.6 | 67.7 | 73.8 | 61.8 |
| CASPER δC (ppm) | 101.2 | 79.1 | 70.8 | 67.8 | 74.0 | 61.8 |
In contrast to the N-terminal domain, C-WbdAO9a was unable to transfer Manp residues to either of the synthetic acceptors (Fig. 10A). No differences were observed between the number of residues transferred by N-WbdAO9a alone and the number transferred in combination with C-WbdAO9a (data not shown). The one-dimensional 1H, gCOSY, and tROESY spectra for the product generated by N-WbdAO9a and C-WbdAO9a combined in the same reaction and N-WbdAO9a alone were indistinguishable (data not shown). These results indicate that the product synthesized by the combined N-WbdAO9a and C-WbdAO9a domains was the same α-(1→2)-linked Manp homopolymer seen in the presence of only N-WbdAO9a. As described above, these same constructs are active in vivo and together catalyze the biosynthesis of the authentic O9a glycan. CD analysis ruled out issues associated with folding of the purified C-WbdAO9a domain as an explanation for its inactivity in vitro (data not shown).
DISCUSSION
The mechanism of the polymerizing WbdA mannosyltransferases from E. coli O8 and O9a is unknown. For WbdAO9a, a bifunctional α-(1→2)-, α-(1→3)-mannosyltransferase, the number of mannosyltransferase activities is correlated with the number of GT4 domains in the protein. This correlation also holds for the trifunctional WbdAO8 enzyme, and the most likely interpretation is that each domain contains an active site confined to one linkage type. Multidomain, bifunctional polymerases with two active sites have been described for hyaluronan (67, 68), chondroitin (68, 69), and heparosan (70–72) biosynthesis.
There is a solid foundation of structure-function data for the CAZy GT4 family of glycosyltransferases. Retaining-enzyme representatives, like WbdC, WbdB, and all but one domain of the WbdA homologs, possess a GT-B fold with a conserved EX7E motif.6 Crystal structures place the EX7E motif within the catalytic site of the enzyme, and the consensus is that the motif is involved in binding of the nucleotide sugar donor (46, 49, 51–53). Available structures show that the two Glu residues interact with hydrogen bond donors comprising the hydroxyl groups of the sugar moiety and ribose moieties of the nucleotide sugar, respectively. The modeled structures of the N- and C-WbdAO9a domains superimposed on PimA suggest that the Glu residues of the WbdAO9a EX7E motifs are likely to be involved in the binding of GDP-Manp. Disruption of these interactions is expected to render the domain inactive. However, there are varying results reported in the literature on the relative importance of one or both Glu residues in the EX7E motif in different glycosyltransferases (46, 52, 58–62, 73). Comparison of solved structures with docked substrates will be necessary to sort out the molecular basis for the different requirements for the EX7E motifs in these enzymes.
In a two-site model, each domain of WbdAO9a must catalyze the formation of two sequential α-(1→2) or α-(1→3) linkages. However, in the absence of a functional C-terminal active site, there is an apparent loss of control over the processivity of Manp transfers catalyzed by N-WbdAO9a; i.e. it adds more than the two sequential α-(1→2)-linked Manp residues seen in the repeat unit synthesized by the full-length protein. The in vitro activity of N-WbdAO9a is entirely consistent with the in vivo activity of the full-length enzyme when the C-terminal active site EX7E motif is mutated. One interpretation of the observed homopolymer is that competition between the active sites or interactions between the two domains in some way regulate the number of residues added. This has been observed in the bifunctional, multidomain KfoC polymerase of E. coli K4, which coordinates polymerization of a capsular polysaccharide with a chondroitin structure through an interaction of peptide sequences located within each domain of the enzyme (74). However, it does not preclude the additional involvement of other regulatory processes, which are generally poorly understood for the enzymes that add a defined number (two or three) of glycosyl residues in a biosynthetic pathway. For example, the Campylobacter jejuni PglH transfers three GalpNAc residues (75), and the number of residues transferred is proposed to be controlled by the relative binding affinities of the enzyme for the growing acceptor (61). The structure of the product synthesized with the synthetic acceptor and the observation that mutations in the EX7E motif in the first WbdAO9a domain completely abolished O-PS production are consistent with the conclusion that the α-(1→2)-mannosyltransferase must precede α-(1→3) transfer in the native assembly system.
The E. coli O9 and O9a O-PSs are distinguished by the presence of an extra α-(1→2)-Manp residue in the O9 repeat unit, suggesting a change to the processivity of N-WbdAO9/O9a (Fig. 1). The O9 and O9a WbdA homologues share 94% identity and 97% similarity at the amino acid level. A single amino acid substitution in the WbdAO9 homologue (C80R) is sufficient for O9 → O9a seroconversion (76). Structural modeling of N-WbdAO9a places Arg80 in close proximity to the active site of N-WbdAO9a (Fig. 3). Based on the model, Arg80 is unlikely to interact with the donor sugar (more than 7 Å away), although it could potentially influence other residues that do interact with the donor. It may also be in a position to form hydrogen bonds with the hydroxyl groups of the Manp residues in the acceptor. This could affect α-(1→2) mannosyltransferase processivity if it involves a control process similar to the one proposed for PglH (61). However, the differences between the activity of N-WbdAO9a in vitro and its role in making the authentic O9a glycan in vivo in the presence of C-WbdAO9a are suggestive of a more complex (and possibly multifactorial) regulatory process. Ultimately, a crystal structure will be necessary to resolve these questions.
Given its activity in vitro, the lack of in vivo activity of N-WbdAO9a is surprising. One possible explanation is that loss of some essential interactions in the absence of C-WbdAO9a compromises in vivo activity. The previous report of the formation of an α-(1→2)-linked mannan by N-WbdAO9a alone (26) could not be replicated here. This observation is difficult to interpret because of the uncertainty of the mutant background. Mapping data suggest that N-WbdAO9a and an N-terminal portion of C-WbdAO9a may still be expressed in the mutant. If correct, the in vivo α-(1→2)- mannosyltransferase activity ascribed previously to the N-WbdAO9a module may require a substantial portion of C-WbdAO9a. Perhaps this region of WbdAO9a is involved in critical interactions with WbdDO9a that are responsible for the localization of the enzyme to the membrane. These interactions may not be essential when using a soluble synthetic acceptor under in vitro conditions.
Although all of the in vivo synthesis, bioinformatics, and mutagenesis data are consistent with C-WbdAO9a possessing α-(1→3)-mannosyltransferase activity, such activity could not be demonstrated in vitro. It is conceivable that C-WbdAO9a activity is dependent on an organized interaction with its N-WbdAO9a partner and that this is compromised in the absence of WbdDO9a. The oligomeric state of WbdAO9a is unknown, but glycosyltransferases often form higher order oligomers (77). Proper oligomerization or multimerization (and thus function) of WbdAO9a may not occur in the absence of the other O9a synthesis components. WbdDO9a forms a trimeric structure (78), so a higher order association of WbdAO9a is possible through its established interaction with WbdD (64). There is precedent for the modulation of glycosyltransferase activity by inter-domain/protein interactions. In the bifunctional, multidomain E. coli K4 KfoC polymerase, disruption of contacts between peptides found within each domain of the enzyme results in a variant with only one active glycosyltransferase domain (74). In contrast, polymerization of the E. coli K5 capsular polysaccharide requires two single domain glycosyltransferases, KfiC and KfiA (79–81). KfiC is inactive in the absence of KfiA, and their interaction is proposed to cause a conformational change in KfiC, allowing it to acquire glycosyltransferase activity (81). In this context, association of the N- and C-terminal domains could change the overall structure of WbdAO9a to produce a bifunctional mannosyltransferase with an active conformation of C-WbdAO9a. Conformational changes (usually upon substrate binding) are a common feature in the mechanism of glycosyltransferases and can involve the movement of flexible loops or the rotation of domains (51, 82, 83). Unlike the K5 system, in vitro function of C-WbdAO9a could not be reconstituted simply by adding N-WbdAO9a, so the situation seems more complex. Until mannosyltransferase activity has been definitively shown for C-WbdAO9a, alternative explanations need to be considered. One possibility is that C-WbdAO9a serves as a regulatory domain that alters the specificity of N-WbdAO9a (enabling it to switch from the transfer of Manp in α-(1→2) to α-(1→3) linkages). In this scenario, only N-WbdAO9a would be catalytically active. A similar phenomenon has been observed in the β4GT-1, the catalytic subunit of lactose synthase, where interaction with the regulatory protein α-lactalbumin alters the specificity for the acceptor substrate (84, 85). However, a parallel situation in WbdAO9a seems unlikely, given the high similarity shared between C-WbdAO9a and active GT4 enzymes as well as the functional requirement for residues in the EX7E motif that are typically associated with substrate binding in systems with solved protein-substrate co-crystal structures (e.g. PimA (46)).
The findings reported here provide a foundation to understand the domain architecture, motifs, and other potentially important residues of the WbdA mannosyltransferases. The data reveal intriguing differences between in vitro and in vivo conditions, reflecting the sensitivity of these enzymes to their reaction environment. Collectively, these results highlight the critical requirement for defined systems to pursue both in vivo and in vitro approaches in studies such as these. The next step will be to determine the structural requirements for interactions between WbdA and WbdD in a functional complex. Understanding the structural context for the flow of alternating activities between the (putative) two catalytic sites is probably dependent on a solved structure for WbdA. Multidomain, bifunctional polymerases with two active sites have been described for hyaluronan (67, 68), chondroitin (68, 69), and heparosan (70–72) biosynthesis; thus, understanding of one system can provide molecular detail about the synthesis of a range of bioactive glycopolymers.
Supplementary Material
Acknowledgments
We thank Dr. J. King for construction of pWQ573 and Dr. B. R. Clarke for construction of pWQ589 and pWQ631. Drs. R. Whittal and J. Zheng (Department of Chemistry Mass Spectrometry Facility, University of Alberta) provided invaluable assistance in obtaining the mass spectra of enzymatically produced products. Drs. D. Hou and C. Liu prepared Acceptors A and B.
This work was supported in part by grants from the Natural Sciences and Engineering Research Council (to C. W. and T. L. L.) and by the Alberta Glycomics Centre (to T. L. L.)

This article contains supplemental Table S1.
B. Henrissat, personal communication.
- O-PS
- O-polysaccharide
- und-PP
- undecaprenol pyrophosphate
- LB
- lysogeny broth
- Man
- mannose
- Manp
- mannopyranose
- GlcpNAc
- N-acetylglucosamine
- MalE
- maltose-binding protein
- gCOSY
- gradient-enhanced correlation spectroscopy
- tROESY
- transverse rotating frame Overhauser enhancement spectroscopy
- gHSQC
- gradient-enhanced heteronuclear single quantum correlation
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Raetz C. R., Whitfield C. (2002) Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Orskov I., Orskov F., Jann B., Jann K. (1977) Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol. Rev. 41, 667–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stenutz R., Weintraub A., Widmalm G. (2006) The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol. Rev. 30, 382–403 [DOI] [PubMed] [Google Scholar]
- 4. Sperandeo P., Dehò G., Polissi A. (2009) The lipopolysaccharide transport system of Gram-negative bacteria. Biochim. Biophys. Acta 1791, 594–602 [DOI] [PubMed] [Google Scholar]
- 5. Cuthbertson L., Kos V., Whitfield C. (2010) ABC transporters involved in export of cell surface glycoconjugates. Microbiol. Mol. Biol. Rev. 74, 341–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greenfield L. K., Whitfield C. (2012) Synthesis of lipopolysaccharide O-antigens by ABC transporter-dependent pathways. Carbohydr. Res. 356, 12–24 [DOI] [PubMed] [Google Scholar]
- 7. Jann K., Kanegasaki S., Goldemann G., Mäkelä P. H. (1979) On the effect of rfe mutation on the biosynthesis of the 08 and 09 antigens of Escherichia coli. Biochem. Biophys. Res. Commun. 86, 1185–1191 [DOI] [PubMed] [Google Scholar]
- 8. Jann K., Goldemann G., Weisgerber C., Wolf-Ullisch C., Kanegasaki S. (1982) Biosynthesis of the O9 antigen of Escherichia coli. Initial reaction and overall mechanism. Eur. J. Biochem. 127, 157–164 [DOI] [PubMed] [Google Scholar]
- 9. Rick P. D., Hubbard G. L., Barr K. (1994) Role of the rfe gene in the synthesis of the O8 antigen in Escherichia coli K-12. J. Bacteriol. 176, 2877–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meier-Dieter U., Starman R., Barr K., Mayer H., Rick P. D. (1990) Biosynthesis of enterobacterial common antigen in Escherichia coli. Biochemical characterization of Tn10 insertion mutants defective in enterobacterial common antigen synthesis. J. Biol. Chem. 265, 13490–13497 [PubMed] [Google Scholar]
- 11. Alexander D. C., Valvano M. A. (1994) Role of the rfe gene in the biosynthesis of the Escherichia coli O7-specific lipopolysaccharide and other O-specific polysaccharides containing N-acetylglucosamine. J. Bacteriol. 176, 7079–7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kido N., Torgov V. I., Sugiyama T., Uchiya K., Sugihara H., Komatsu T., Kato N., Jann K. (1995) Expression of the O9 polysaccharide of Escherichia coli. Sequencing of the E. coli O9 rfb gene cluster, characterization of mannosyl transferases, and evidence for an ATP-binding cassette transport system. J. Bacteriol. 177, 2178–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenfield L. K., Richards M., Li J., Lowary T. D., Wakarchuk W. W., Whitfield C. (August 8, 2012) Biosynthesis of the polymannose lipopolysaccharide O-antigens from Escherichia coli serotypes O8 and O9a requires a unique combination of single- and multiple-active site mannosyltransferases J. Biol. Chem. 10.1074/jbc.M112.401000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clarke B. R., Cuthbertson L., Whitfield C. (2004) Nonreducing terminal modifications determine the chain length of polymannose O-antigens of Escherichia coli and couple chain termination to polymer export via an ATP-binding cassette transporter. J. Biol. Chem. 279, 35709–35718 [DOI] [PubMed] [Google Scholar]
- 15. Clarke B. R., Richards M. R., Greenfield L. K., Hou D., Lowary T. L., Whitfield C. (2011) In vitro reconstruction of the chain termination reaction in biosynthesis of the Escherichia coli O9a O-polysaccharide. The chain length regulator, WbdD, catalyzes the addition of methyl phosphate to the non-reducing terminus of the growing glycan. J. Biol. Chem. 286, 41391–41401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cuthbertson L., Kimber M. S., Whitfield C. (2007) Substrate binding by a bacterial ABC transporter involved in polysaccharide export. Proc. Natl. Acad. Sci. U.S.A. 104, 19529–19534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reske K., Jann K. (1972) The O8 antigen of Escherichia coli. Structure of the polysaccharide chain. Eur. J. Biochem. 31, 320–328 [DOI] [PubMed] [Google Scholar]
- 18. Prehm P., Jann B., Jann K. (1976) The O9 antigen of Escherichia coli. Structure of the polysaccharide chain. Eur. J. Biochem. 67, 53–56 [DOI] [PubMed] [Google Scholar]
- 19. Jansson P. E., Lönngren J., Widmalm G., Leontein K., Slettengren K., Svenson S. B., Wrangsell G., Dell A., Tiller P. R. (1985) Structural studies of the O-antigen polysaccharides of Klebsiella O5 and Escherichia coli O8. Carbohydr. Res. 145, 59–66 [DOI] [PubMed] [Google Scholar]
- 20. Parolis L. A., Parolis H., Dutton G. G. (1986) Structural studies of the O-antigen polysaccharide of Escherichia coli 09a. Carbohydr. Res. 155, 272–276 [DOI] [PubMed] [Google Scholar]
- 21. Saeki A., Kido N., Sugiyama T., Ohta M., Iwashita T., Uchiya K., Kato N. (1993) Isolation of rfb gene clusters directing the synthesis of O polysaccharides consisting of mannose homopolymers and serological analysis of lipopolysaccharides. Microbiol. Immunol. 37, 601–606 [DOI] [PubMed] [Google Scholar]
- 22. Vinogradov E., Frirdich E., MacLean L. L., Perry M. B., Petersen B. O., Duus J. Ø., Whitfield C. (2002) Structures of lipopolysaccharides from Klebsiella pneumoniae. Elucidation of the structure of the linkage region between core and polysaccharide O chain and identification of the residues at the non-reducing termini of the O chains. J. Biol. Chem. 277, 25070–25081 [DOI] [PubMed] [Google Scholar]
- 23. Kubler-Kielb J., Whitfield C., Katzenellenbogen E., Vinogradov E. (2012) Identification of the methyl phosphate substituent at the non-reducing terminal mannose residue of the O-specific polysaccharides of Klebsiella pneumoniae O3, Hafnia alvei PCM 1223, and Escherichia coli O9/O9a LPS. Carbohydr. Res. 347, 186–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marchler-Bauer A., Lu S., Anderson J. B., Chitsaz F., Derbyshire M. K., DeWeese-Scott C., Fong J. H., Geer L. Y., Geer R. C., Gonzales N. R. (2011) CDD. A conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 39, D225–D229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cantarel B. L., Coutinho P. M., Rancurel C., Bernard T., Lombard V., Henrissat B. (2009) The Carbohydrate-Active EnZymes database (CAZy). An expert resource for glycogenomics. Nucleic Acids Res. 37, D233–D238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kido N., Sugiyama T., Yokochi T., Kobayashi H., Okawa Y. (1998) Synthesis of Escherichia coli O9a polysaccharide requires the participation of two domains of WbdA, a mannosyltransferase encoded within the wb* gene cluster. Mol. Microbiol. 27, 1213–1221 [DOI] [PubMed] [Google Scholar]
- 27. Miller J. H. (1972) Experiments in Molecular Genetics, pp. 432–433, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 28. Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R. (2007) ClustalW and ClustalX version 2.0. Bioinformatics 23, 2947–2948 [DOI] [PubMed] [Google Scholar]
- 29. Goujon M., McWilliam H., Li W., Valentin F., Squizzato S., Paern J., Lopez R. (2010) A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 38, W695–W699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cole C., Barber J. D., Barton G. J. (2008) The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 36, W197–W201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ouali M., King R. D. (2000) Cascaded multiple classifiers for secondary structure prediction. Protein Sci. 9, 1162–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheng J., Randall A. Z., Sweredoski M. J., Baldi P. (2005) SCRATCH. A protein structure and structural feature prediction server. Nucleic Acids Res. 33, W72–W76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jones D. T. (1999) Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292, 195–202 [DOI] [PubMed] [Google Scholar]
- 34. Buchan D. W., Ward S. M., Lobley A. E., Nugent T. C., Bryson K., Jones D. T. (2010) Protein annotation and modeling servers at University College London. Nucleic Acids Res. 38, W563–W568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marchler-Bauer A., Bryant S. H. (2004) CD-Search. Protein domain annotations on the fly. Nucleic Acids Res. 32, W327–W331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marchler-Bauer A., Anderson J. B., Chitsaz F., Derbyshire M. K., DeWeese-Scott C., Fong J. H., Geer L. Y., Geer R. C., Gonzales N. R., Gwadz M., He S., Hurwitz D. I., Jackson J. D., Ke Z., Lanczycki C. J., Liebert C. A., Liu C., Lu F., Lu S., Marchler G. H., Mullokandov M., Song J. S., Tasneem A., Thanki N., Yamashita R. A., Zhang D., Zhang N., Bryant S. H. (2009) CDD. Specific functional annotation with the Conserved Domain Database. Nucleic Acids Res. 37, D205–D210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kelley L. A., Sternberg M. J. (2009) Protein structure prediction on the Web. A case study using the Phyre server. Nat. Protoc. 4, 363–371 [DOI] [PubMed] [Google Scholar]
- 38. Gasteiger E., Hoogland C., Gattiker A., Duvaud S., Wilkins M. R., Appel R. D., Bairoch A. (2005) in The Proteomics Protocols Handbook (Walker J. M., eds) pp. 571–607, Humana Press, Totowa, NJ [Google Scholar]
- 39. Westphal O., Jann J. A. (1965) in Methods in Carbohydrate Chemistry (Whister R. C., ed) Vol. 5, pp. 83–91, Academic Press, Inc., New York [Google Scholar]
- 40. Liu C., Skogman F., Cai Y., Lowary T. L. (2007) Synthesis of the “primer-adaptor” trisaccharide moiety of Escherichia coli O8, O9, and O9a lipopolysaccharide. Carbohydr. Res. 342, 2818–2825 [DOI] [PubMed] [Google Scholar]
- 41. Hou D., Skogman F., Lowary T. L. (2008) Synthesis of 8-azidooctyl glycoside derivatives of the O-chain repeating unit of Escherichia coli O9a lipopolysaccharide and a methylated analog. Carbohydr. Res. 343, 1778–1789 [DOI] [PubMed] [Google Scholar]
- 42. Hitchcock P. J., Brown T. M. (1983) Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154, 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 44. Tsai C. M., Frasch C. E. (1982) A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119, 115–119 [DOI] [PubMed] [Google Scholar]
- 45. Korduláková J., Gilleron M., Mikusova K., Puzo G., Brennan P. J., Gicquel B., Jackson M. (2002) Definition of the first mannosylation step in phosphatidylinositol mannoside synthesis. PimA is essential for growth of mycobacteria. J. Biol. Chem. 277, 31335–31344 [DOI] [PubMed] [Google Scholar]
- 46. Guerin M. E., Kordulakova J., Schaeffer F., Svetlikova Z., Buschiazzo A., Giganti D., Gicquel B., Mikusova K., Jackson M., Alzari P. M. (2007) Molecular recognition and interfacial catalysis by the essential phosphatidylinositol mannosyltransferase PimA from mycobacteria. J. Biol. Chem. 282, 20705–20714 [DOI] [PubMed] [Google Scholar]
- 47. Geremia R. A., Petroni E. A., Ielpi L., Henrissat B. (1996) Toward a classification of glycosyltransferases based on amino acid sequence similarities. Prokaryotic α-mannosyltransferases. Biochem. J. 318, 133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ruane K. M., Davies G. J., Martinez-Fleites C. (2008) Crystal structure of a family GT4 glycosyltransferase from Bacillus anthracis ORF BA1558. Proteins 73, 784–787 [DOI] [PubMed] [Google Scholar]
- 49. Martinez-Fleites C., Proctor M., Roberts S., Bolam D. N., Gilbert H. J., Davies G. J. (2006) Insights into the synthesis of lipopolysaccharide and antibiotics through the structures of two retaining glycosyltransferases from family GT4. Chem. Biol. 13, 1143–1152 [DOI] [PubMed] [Google Scholar]
- 50. Chua T. K., Bujnicki J. M., Tan T. C., Huynh F., Patel B. K., Sivaraman J. (2008) The structure of sucrose phosphate synthase from Halothermothrix orenii reveals its mechanism of action and binding mode. Plant Cell 20, 1059–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vetting M. W., Frantom P. A., Blanchard J. S. (2008) Structural and enzymatic analysis of MshA from Corynebacterium glutamicum. Substrate-assisted catalysis. J. Biol. Chem. 283, 15834–15844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Batt S. M., Jabeen T., Mishra A. K., Veerapen N., Krumbach K., Eggeling L., Besra G. S., Fütterer K. (2010) Acceptor substrate discrimination in phosphatidyl-myo-inositol mannoside synthesis. Structural and mutational analysis of mannosyltransferase Corynebacterium glutamicum PimB′. J. Biol. Chem. 285, 37741–37752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parsonage D., Newton G. L., Holder R. C., Wallace B. D., Paige C., Hamilton C. J., Dos Santos P. C., Redinbo M. R., Reid S. D., Claiborne A. (2010) Characterization of the N-acetyl-α-d-glucosaminyl l-malate synthase and deacetylase functions for bacillithiol biosynthesis in Bacillus anthracis. Biochemistry 49, 8398–8414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Woo E. J., Ryu S. I., Song H. N., Jung T. Y., Yeon S. M., Lee H. A., Park B. C., Park K. H., Lee S. B. (2010) Structural insights on the new mechanism of trehalose synthesis by trehalose synthase TreT from Pyrococcus horikoshii. J. Mol. Biol. 404, 247–259 [DOI] [PubMed] [Google Scholar]
- 55. Xiang Y., Baxa U., Zhang Y., Steven A. C., Lewis G. L., Van Etten J. L., Rossmann M. G. (2010) Crystal structure of a virus-encoded putative glycosyltransferase. J. Virol. 84, 12265–12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee S. J., Lee B. I., Suh S. W. (2011) Crystal structure of the catalytic domain of cholesterol-α-glucosyltransferase from Helicobacter pylori. Proteins 79, 2321–2326 [DOI] [PubMed] [Google Scholar]
- 57. Zheng Y., Anderson S., Zhang Y., Garavito R. M. (2011) The structure of sucrose synthase-1 from Arabidopsis thaliana and its functional implications. J. Biol. Chem. 286, 36108–36118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abdian P. L., Lellouch A. C., Gautier C., Ielpi L., Geremia R. A. (2000) Identification of essential amino acids in the bacterial α-mannosyltransferase aceA. J. Biol. Chem. 275, 40568–40575 [DOI] [PubMed] [Google Scholar]
- 59. Cid E., Gomis R. R., Geremia R. A., Guinovart J. J., Ferrer J. C. (2000) Identification of two essential glutamic acid residues in glycogen synthase. J. Biol. Chem. 275, 33614–33621 [DOI] [PubMed] [Google Scholar]
- 60. Kostova Z., Yan B. C., Vainauskas S., Schwartz R., Menon A. K., Orlean P. (2003) Comparative importance in vivo of conserved glutamate residues in the EX7E motif retaining glycosyltransferase Gpi3p, the UDP-GlcNAc-binding subunit of the first enzyme in glycosylphosphatidylinositol assembly. Eur. J. Biochem. 270, 4507–4514 [DOI] [PubMed] [Google Scholar]
- 61. Troutman J. M., Imperiali B. (2009) Campylobacter jejuni PglH is a single active site processive polymerase that utilizes product inhibition to limit sequential glycosyl transfer reactions. Biochemistry 48, 2807–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Absmanner B., Schmeiser V., Kämpf M., Lehle L. (2010) Biochemical characterization, membrane association, and identification of amino acids essential for the function of Alg11 from Saccharomyces cerevisiae, an α1,2-mannosyltransferase catalyzing two sequential glycosylation steps in the formation of the lipid-linked core oligosaccharide. Biochem. J. 426, 205–217 [DOI] [PubMed] [Google Scholar]
- 63. Shatzman A. R., Gross M. S., Rosenberg M. (2001) in Current Protocols in Molecular Biology, pp. 11:16.3.1–6.3.11, John Wiley & Sons, Inc., New York [Google Scholar]
- 64. Clarke B. R., Greenfield L. K., Bouwman C., Whitfield C. (2009) Coordination of polymerization, chain termination, and export in assembly of the Escherichia coli lipopolysaccharide O9a antigen in an ATP-binding cassette transporter-dependent pathway. J. Biol. Chem. 284, 30662–30672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jansson P. E., Stenutz R., Widmalm G. (2006) Sequence determination of oligosaccharides and regular polysaccharides using NMR spectroscopy and a novel Web-based version of the computer program CASPER. Carbohydr. Res. 341, 1003–1010 [DOI] [PubMed] [Google Scholar]
- 66. Lundborg M., Widmalm G. (2011) Structure analysis of glycans by NMR chemical shift prediction. Anal. Chem. 83, 1514–1517 [DOI] [PubMed] [Google Scholar]
- 67. Jing W., DeAngelis P. L. (2000) Dissection of the two transferase activities of the Pasteurella multocida hyaluronan synthase. Two active sites exist in one polypeptide. Glycobiology 10, 883–889 [DOI] [PubMed] [Google Scholar]
- 68. Jing W., DeAngelis P. L. (2003) Analysis of the two active sites of the hyaluronan synthase and the chondroitin synthase of Pasteurella multocida. Glycobiology 13, 661–671 [DOI] [PubMed] [Google Scholar]
- 69. Sobhany M., Kakuta Y., Sugiura N., Kimata K., Negishi M. (2008) The chondroitin polymerase K4CP and the molecular mechanism of selective bindings of donor substrates to two active sites. J. Biol. Chem. 283, 32328–32333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kane T. A., White C. L., DeAngelis P. L. (2006) Functional characterization of PmHS1, a Pasteurella multocida heparosan synthase. J. Biol. Chem. 281, 33192–33197 [DOI] [PubMed] [Google Scholar]
- 71. Chavaroche A. A., van den Broek L. A., Boeriu C., Eggink G. (2012) Synthesis of heparosan oligosaccharides by Pasteurella multocida PmHS2 single-action transferases. Appl. Microbiol. Biotechnol. 95, 1199–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chavaroche A. A., van den Broek L. A., Springer J., Boeriu C., Eggink G. (2011) Analysis of the polymerization initiation and activity of Pasteurella multocida heparosan synthase PmHS2, an enzyme with glycosyltransferase and UDP-sugar hydrolase activity. J. Biol. Chem. 286, 1777–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kämpf M., Absmanner B., Schwarz M., Lehle L. (2009) Biochemical characterization and membrane topology of Alg2 from Saccharomyces cerevisiae as a bifunctional α1,3- and 1,6-mannosyltransferase involved in lipid-linked oligosaccharide biosynthesis. J. Biol. Chem. 284, 11900–11912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sobhany M., Kakuta Y., Sugiura N., Kimata K., Negishi M. (August 30, 2012) The structural basis for a coordinated reaction catalyzed by a bifunctional glycosyltransferase in chondroitin biosynthesis. J. Biol. Chem. 10.1074/jbc.M112.375873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Glover K. J., Weerapana E., Imperiali B. (2005) In vitro assembly of the undecaprenyl pyrophosphate-linked heptasaccharide for prokaryotic N-linked glycosylation. Proc. Natl. Acad. Sci. U.S.A. 102, 14255–14259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kido N., Kobayashi H. (2000) A single amino acid substitution in a mannosyltransferase, WbdA, converts the Escherichia coli O9 polysaccharide into O9a. Generation of a new O-serotype group. J. Bacteriol. 182, 2567–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hashimoto K., Madej T., Bryant S. H., Panchenko A. R. (2010) Functional states of homooligomers. Insights from the evolution of glycosyltransferases. J. Mol. Biol. 399, 196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hagelueken G., Huang H., Clarke B. R., Lebl T., Whitfield C., Naismith J. H. (2012) Mol. Microbiol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Griffiths G., Cook N. J., Gottfridson E., Lind T., Lidholt K., Roberts I. S. (1998) Characterization of the glycosyltransferase enzyme from the Escherichia coli K5 capsule gene cluster and identification and characterization of the glucuronyl active site. J. Biol. Chem. 273, 11752–11757 [DOI] [PubMed] [Google Scholar]
- 80. Hodson N., Griffiths G., Cook N., Pourhossein M., Gottfridson E., Lind T., Lidholt K., Roberts I. S. (2000) Identification that KfiA, a protein essential for the biosynthesis of the Escherichia coli K5 capsular polysaccharide, is an α-UDP-GlcNAc glycosyltransferase. The formation of a membrane-associated K5 biosynthetic complex requires KfiA, KfiB, and KfiC. J. Biol. Chem. 275, 27311–27315 [DOI] [PubMed] [Google Scholar]
- 81. Sugiura N., Baba Y., Kawaguchi Y., Iwatani T., Suzuki K., Kusakabe T., Yamagishi K., Kimata K., Kakuta Y., Watanabe H. (2010) Glucuronyltransferase activity of KfiC from Escherichia coli strain K5 requires association of KfiA. KfiC and KfiA are essential enzymes for production of K5 polysaccharide, N-acetylheparosan. J. Biol. Chem. 285, 1597–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Qasba P. K., Ramakrishnan B., Boeggeman E. (2005) Substrate-induced conformational changes in glycosyltransferases. Trends Biochem. Sci. 30, 53–62 [DOI] [PubMed] [Google Scholar]
- 83. Breton C., Snajdrová L., Jeanneau C., Koca J., Imberty A. (2006) Structures and mechanisms of glycosyltransferases. Glycobiology 16, 29R–37R [DOI] [PubMed] [Google Scholar]
- 84. Brew K., Vanaman T. C., Hill R. L. (1968) The role of α-lactalbumin and the A protein in lactose synthetase. A unique mechanism for the control of a biological reaction. Proc. Natl. Acad. Sci. U.S.A. 59, 491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schanbacher F. L., Ebner K. E. (1970) Galactosyltransferase acceptor specificity of the lactose synthetase A protein. J. Biol. Chem. 245, 5057–5061 [PubMed] [Google Scholar]
- 86. Guzman L. M., Belin D., Carson M. J., Beckwith J. (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177, 4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cieslewicz M., Vimr E. (1997) Reduced polysialic acid capsule expression in Escherichia coli K1 mutants with chromosomal defects in kpsF. Mol. Microbiol. 26, 237–249 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.