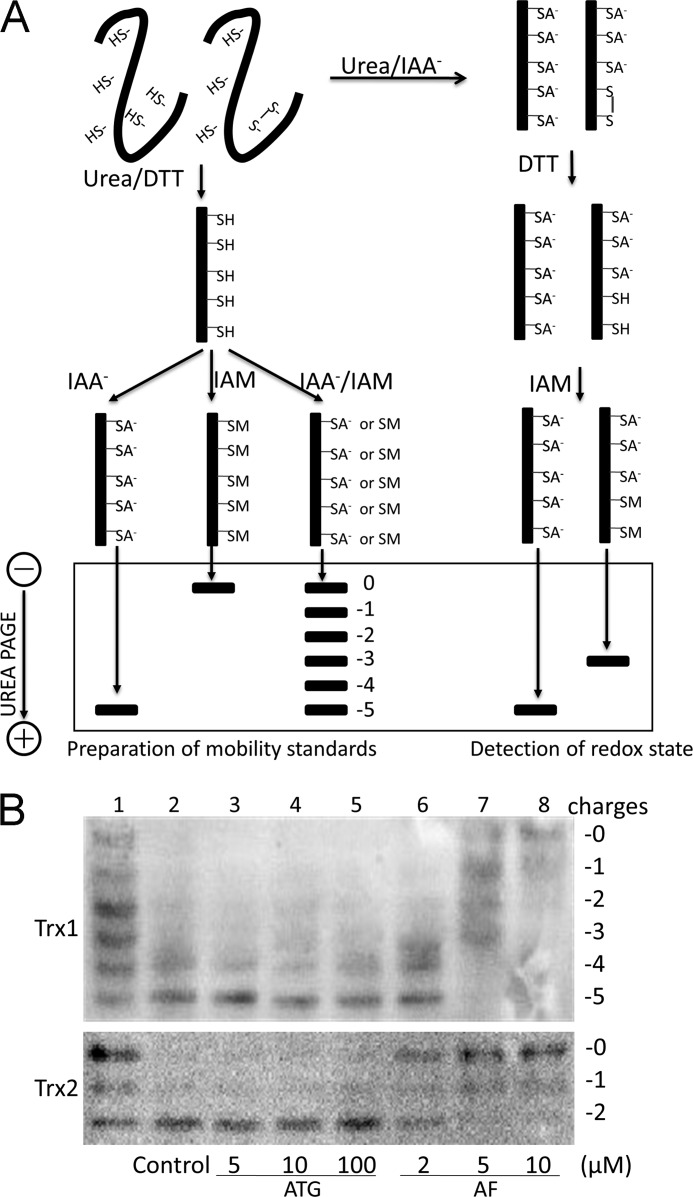
FIGURE 2.
Redox state of Trx1/2 in HeLa cells exposed to ATG and AF. A, principle of redox Western blot analysis. To prepare mobility standards, cell lysates are denatured with urea and fully reduced with DTT. Then, varying molar ratios of IAA to IAM are incubated with the reduced thioredoxin containing five cysteines, producing six protein isoforms with introduced number of acidic carboxymethyl thiol adducts (-SA−) and neutral amidomethyl thiol adducts (-SM). During urea-PAGE, the ionized -SA− group resulted in faster protein migration toward the anode. Therefore, the six isoforms are separated and used as a mobility standard for representing the number of -SA−. To determine the redox state of thioredoxin in compound-treated cells, HeLa cells were lysed in urea lysis buffer containing IAA. After the free thiols of thioredoxin were alkylated by IAA, cell lysates were precipitated by ice-cold acetone-HCl. The precipitate was washed with ice-cold acetone-HCl two more times to remove excess IAA. Then the precipitate was resuspended in urea lysis buffer containing DTT to reduce thioredoxin containing an inter- or intramolecular disulfide bridge. The free thiols of thioredoxin were then alkylated by IAM. The alkylated thioredoxins in cell lysates were separated according to the charge amount. B, HeLa cells were treated by indicated concentrations of ATG (lanes 3-5) or AF (lanes 6-8) for 24 h, and then the redox states of Trx1 and Trx2 in HeLa cells were detected with redox state Western blot analysis under steady-state conditions. Lane 1, mobility standards. Lane 2, HeLa cells without treatment.