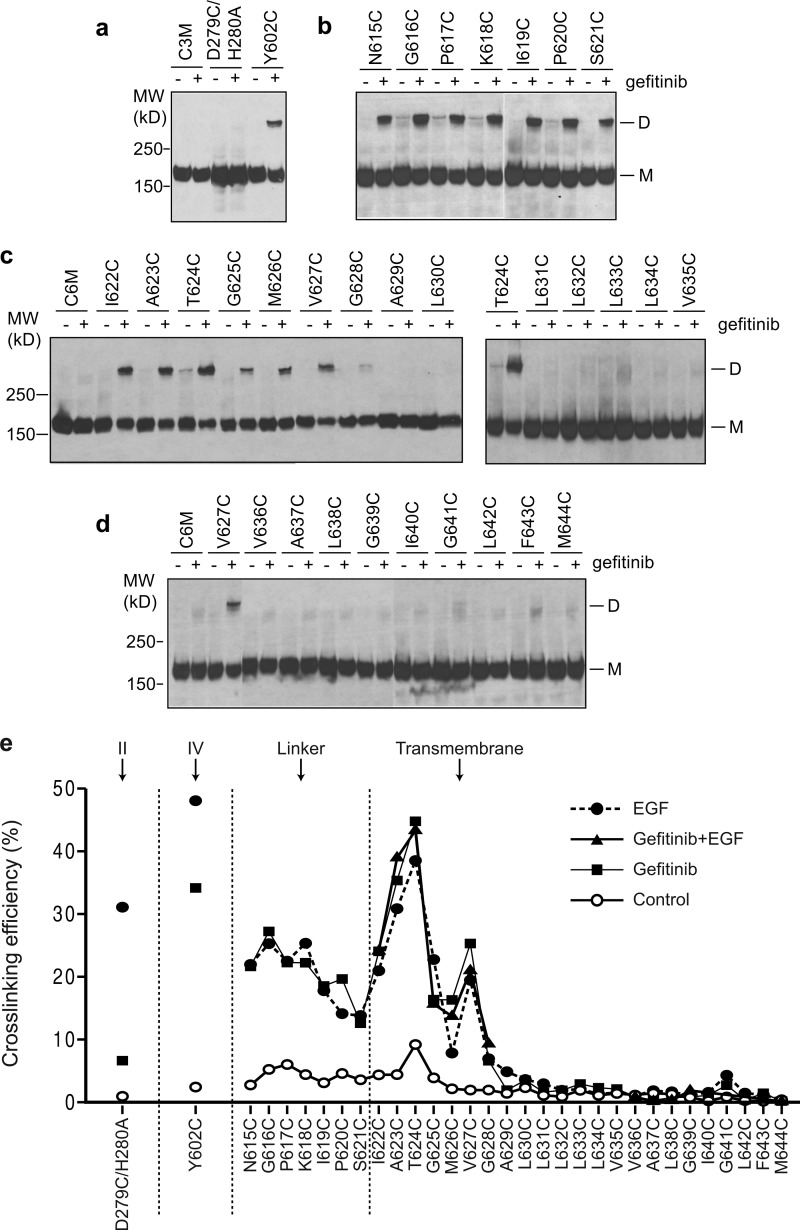
FIGURE 1.
Disulfide cross-linking of EGFR cysteine mutants in Ba/F3 transfectants treated with or without EGFR-specific kinase inhibitor, gefitinib. The cells were treated with 10 μm gefitinib or Me2SO control for 45 min. The lysates were subjected to nonreducing SDS 5% PAGE and Western blotting with protein C antibody to detect EGFR protein. a, cysteine substitutions in the domain II (D279C/H280A) and domain IV (Y602C) interfaces in the crystal structure of liganded dimer. b, linker residues. c and d, TM domain residues. e, comparison of disulfide cross-linking induced by gefitinib, EGF, and gefitinib in combination with EGF. EGF-induced cross-linking data were published previously (9). MW, molecular mass; D, dimeric; M, monomeric.