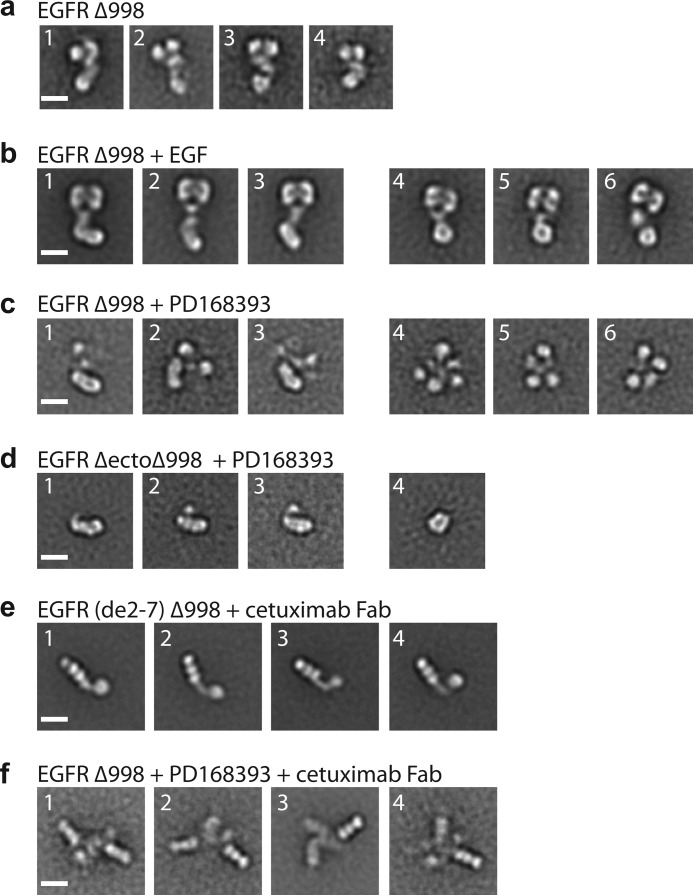
FIGURE 5.
Kinase inhibitor promotes formation of the asymmetric kinase dimer with ectodomain conformation distinct from liganded dimer. a, representative class averages of unliganded, monomeric EGFR Δ998. b, representative class averages of EGF-bound, dimeric EGFR Δ998, in which symmetric ectodomain dimers are linked to asymmetric kinase dimers (subpanels 1–3) or symmetric kinase dimers (subpanels 4–6). c, representative class averages of PD168393-bound EGFR Δ998 with asymmetric kinase dimer (subpanels 1–3) or less interpretable densities (subpanels 4–6). d, representative class averages of PD168393-bound EGFR ΔectoΔ998 with asymmetric kinase dimer (subpanels 1–3) or symmetric kinase dimer (subpanel 4). e, representative class averages of EGFR(de2–7)Δ998 in complex with cetuximab Fab. f, representative class averages of EGFR Δ998 with PD168393 in complex with cetuximab Fab. Scale bars, 10 nm.