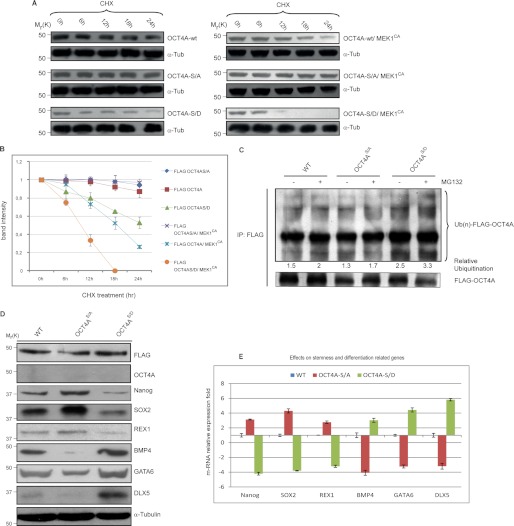
FIGURE 5.
Ser-111 phosphorylation enhances OCT4A proteasome-dependent degradation. A, HeLa cells were transfected with indicated plasmids. At 24–48 h after transfection, cells were treated with 15 μg/ml CHX. At the indicated time points, whole cell lysates were prepared, and immunoblots were probed with the indicated antibodies. OCT4A-S/A, OCT4A(S111A); OCT4A-S/D, OCT4A(S111D). B, FLAG-OCT4A band intensity was normalized to α-tubulin (α-Tub) and then normalized to the t = 0 controls. Results are shown as means ± S.D. for three independent sets of experiments. C, HeLa cells transfected with the WT or the S111A or S111D mutant were lysed and immunoprecipitated (IP) using anti-FLAG beads. Immunoblotting was performed using anti-ubiquitin (Ub) and anti-FLAG antibodies. D, OCT4A-silenced NTera2 cells transfected with the WT or the S111A or S111D mutant were reacted with anti-FLAG, anti-OCT4A, anti-Nanog, anti-SOX2, anti-REX1, anti-BMP4, anti-GATA6, anti-DLX5, and anti-α-tubulin antibodies. E, the same stemness (Nanog, SOX2, and REX1) and differentiation (BMP4, GATA6, and DLX5) markers were tested by real-time PCR. Data were normalized to RNA polymerase II and are shown as means ± S.D. for three independent sets of experiments.