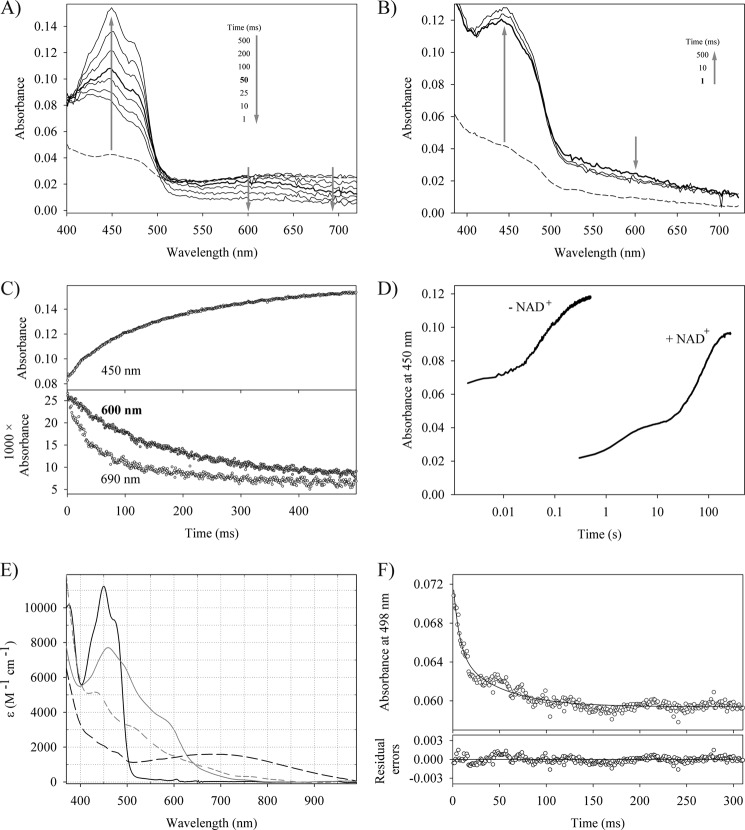
FIGURE 2.
Oxidative half-reaction of reductaseTOL with various electron acceptors. Time-dependent spectral changes of 15 μm NADH-reduced reductaseTOL reacting with 30 μm potassium ferricyanide (A) and 8 μm dithionite-reduced reductaseTOL reacting with 20 μm potassium ferricyanide (B). The dashed line represents the spectrum of reduced reductaseTOL, and arrows indicate the direction of absorbance changes. C, time-dependent absorbance change at 450, 600, and 690 nm for the reaction shown in A. D, time-dependent change in absorption at 450 nm when reduced reductaseTOL is mixed with an air-saturated solution. The trace labeled − NAD+ shows the reoxidation of dithionite-reduced reductaseTOL, whereas that labeled + NAD+ shows the recorded trace for NADH-reduced reductaseTOL where the NAD+-reductaseTOLCT is found in solution. E, absorption spectra of oxidized reductaseTOL (solid black line), oxidized ferredoxinTOL (solid gray line), NAD+-reductaseTOLCT (dashed black line), and sodium dithionite-reduced ferredoxinTOL (dashed gray line). F, stopped-flow trace at 498 nm for the reaction of 19.8 μm reductaseTOL with 10 μm ferredoxinTOL. At this wavelength, the kinetics of the electron transfer to the iron-sulfur cluster of ferredoxinTOL is observed without significant contributions from the flavin species. The solid line shows a double exponential fit to the data, yielding observed rate constants of 153.8 and 21.4 s−1 and amplitudes of 0.0075 and 0.0056, respectively.