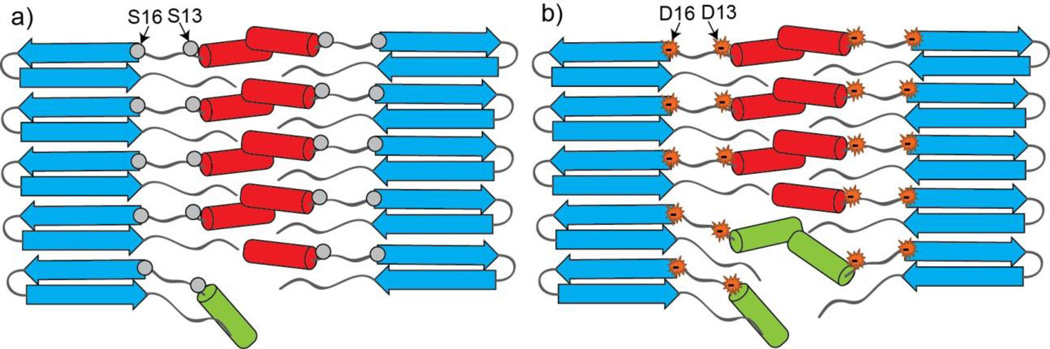
Figure 8.
A model for the htt exon1-like fibril structure, integrating observations from ssNMR, EM and trypsin cleavage. In the figures, polyQ β-sheets are in blue, more rigid httNT α-helices are in red, and more mobile httNT α-helices are in green. Positions 13 and 16 are in a segment lacking secondary structure 27. The figure shows how increased mobility (green helices) caused by charge repulsion (b) leads to greater exposure of httNT sequences to trypsin access compared to more stably packed WT httNT (a). In both WT and mutated httNT, the bulk of the httNT helices (red) remain stably involved in structure and detectible by MAS ssNMR.