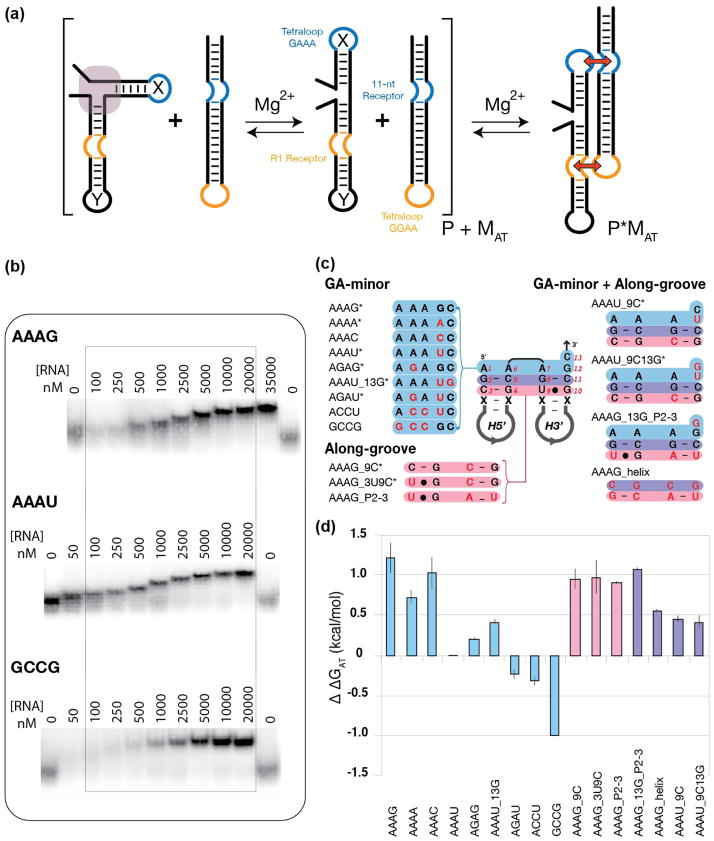
Figure 2.
Probing the thermodynamics of minimal RA variant constructs based on tectoRNA assembly attenuation. (a) Schematic illustrating the basic experimental design strategy: RNA molecules containing a GAAA tetraloop, an R1 receptor, and a variant RA motif sequence signatures at the junction between the 5′ and the 3′ hairpin (designated helix X and Y respectively) were evaluated based on their ability to bind to a probe molecule possessing an 11-nt receptor and a GGAA tetraloop. Stronger attenuation corresponds to a more stable RA motif. (b) Sample native PAGE (1x TB) gel-shift assays of titration experiments used to determine relative equilibrium dissociation constant (Kd) in TB 1x at 15mM Mg2+ at 10°C. (c) List of the RA variants tested in the minimal tectoRNA system. The RA sequence in the middle corresponds to the AAAG construct. Construct variants are named after the sequence of their GA minor components (positions 1, 6, 7, 12, and 13 in blue) and the sequence variations (in red) localized in their along-groove component (in pink). Asterisks indicate natural ribosomal RA sequences. (d) Apparent free energy of attenuation of heterodimer formation (ΔΔGAT) for all minimal RA constructs, referenced to the AAAU construct.