Abstract
The sensory outer segments (OS) of vertebrate retinal photoreceptors, which detect photons of light, resemble the distal segments of C. elegans sensory cilia, which detect chemical ligands that influence the chemotactic movements of the animal. Based on fluorescence microscopy assays performed in sensory cilia of living, transgenic “wild type” and mutant C. elegans, combined with in vitro motility assays using purified motors, we have proposed that two types of kinesin-2 motor, heterotrimeric kinesin-II and homodimeric OSM-3, cooperate to build amphid and phasmid sensory cilia on chemosensory neurons. Specifically, we propose that these motors function together in a redundant manner to build the axoneme core (aka middle segments (MS)), whereas OSM-3 alone serves to build the distal segments (DS). Furthermore, our data suggest that these motors accomplish this by driving two sequential steps of anterograde transport of cargoes consisting of IFT-particles, retrograde dynein motors, and ciliary tubulin subunits, from the transition zone to the tips of the axonemal microtubules (MTs). Homologs of kinesin-II (KIF3) and OSM-3 (KIF17) are also proposed to contribute to the assembly of vertebrate photoreceptors, although how they do so is currently unclear. Here I review our work on kinesin-2 motors, intraflagellar transport (IFT) and cilium biogenesis in C. elegans sensory cilia, and comment on its possible relevance to current research on vertebrate photoreceptor cilia assembly and function.
1. Introduction
Sensory (aka primary) cilia are currently recognized as playing important roles in most eukaryotic cells, by serving as antenna-like signaling platforms that concentrate signal transducing molecules, detect extracellular sensory stimuli and transduce them into signals that are transmitted to the cytoplasm or nucleus to control many cellular and developmental processes (Ishikawa and Marshall, 2011). For example, in the vertebrate retina, rod and cone photoreceptors are specialized neurons whose outer segments are elaborate sensory cilia that contain stacks of membranes enriched in opsins which detect photons of light, together with associated phototransduction molecules e.g. the heterotrimeric G protein, transducin, which activates cGMP phosphodiesterase to reduce cGMP levels and close cyclic nucleotide gated (CNG) channels in the cilium membrane, plus arrestin which inactivates rhodopsin following its photoactivation (Insinna and Besharse, 2008; Yau and Hardie, 2009). Similarly, in the C. elegans nervous system, sensory cilia occur on the dendritic endings of chemosensory neurons where they concentrate various chemoreceptors and associated signaling molecules that detect chemicals in the environment and send signals via networks of inter- and motor-neurons to the body wall musculature to control the chemotactic movements of the animal (Bargmann, 1997; Inglis et al., 2007; Perkins et al., 1986) (Fig. 1). In both these types of sensory cilia, so-called “distal singlets” are thought to play significant roles in sensory signaling because their specific loss, for example in osm-3 mutants (Perkins et al., 1986), leads to the failure to detect and respond to environmental chemical stimuli, but exactly how they contribute to cilium-based signaling is unclear.
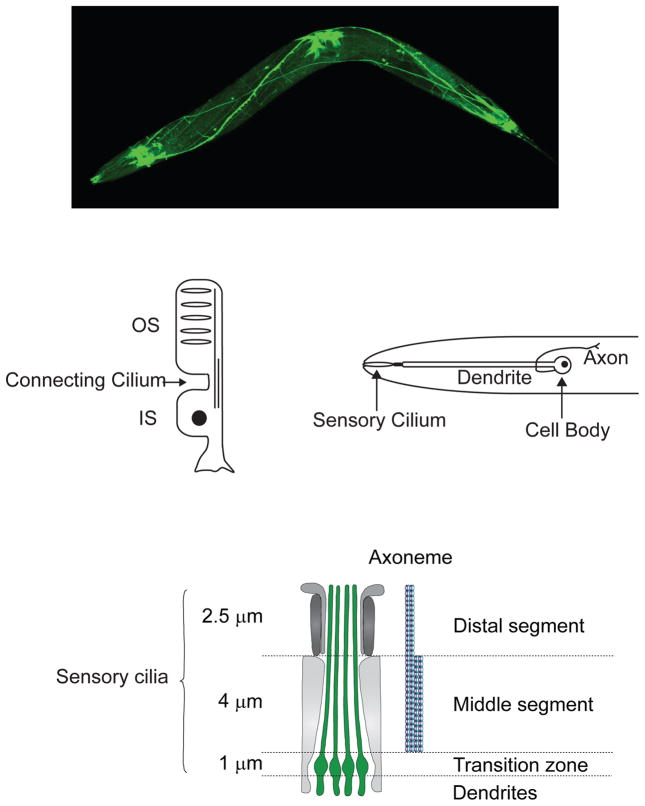
Figure 1.
Sensory Cilia in the C. elegans nervous system and their relationship to vertebrate photoreceptors. Upper panel, living C. elegans expressing GFP to illuminate the nervous system, including sensory cilia forming the “nose” of the animal on the extreme left. Middle panel, drawing comparing vertebrate photoreceptors (left) with C. elegans sensory cilia (right). Lower panel, cartoon of section through a bundle of amphid channel cilia showing the longitudinal differentiation of amphid sensory cilia into middle segments containing 9 doublet MTs and distal segments containing 9 singlet MTs (Evans et al., 2006).
The assembly and maintenance of rod and cone photoreceptor outer segments (OS) requires rapid trafficking of a variety of building blocks from the endoplasmic reticulum/golgi apparatus/trans golgi network in the IS, through the connecting cilium, to the outer segment. Similarly, in C. elegans chemosensory neurons, sensory ciliary building blocks are thought to be synthesized in the cell body and trafficked along the dendrite, through the transition zone, and along the ciliary axoneme for incorporation at the distal tip. It seems likely that various intracellular transport mechanisms, including diffusion, actin-based transport and MT-based transport contribute to these processes (Calvert et al., 2006; Insinna and Besharse, 2008; Williams, 2002). Among these, kinesin-2 dependent intraflagellar transport (IFT) is currently drawing much attention and is the focus of the current presentation.
2. Kinesin-2 motors, Intraflagellar Transport and Ciliogenesis
Our own work in this area began with the fortuitous discovery of a new form of kinesin. Eukaryotic cells are now known to contain multiple, functionally diverse kinesin motors, that are organized into 14 families with some transporting cargoes towards the plus or towards the minus ends of MTs, and others serving as MT polymerases or depolymerases (Lawrence et al., 2004). The founding member of this superfamily, kinesin-1, was isolated as a heterotetrameric fast axonal organelle transport motor consisting of 2 identical motor subunits (KHC) and 2 “light chains” (KLC) via microtubule affinity purification from neuronal cell extracts (Vale et al., 1985)
Subsequently, in a search for motors that mediate mitosis and chromosome segregation, we purified a different, heterotrimeric plus-end-directed MT-based motor named kinesin-2, consisting of 2 distinct KHC-related motor subunits and an accessory “KAP” subunit, from echinoderm egg/embryo extracts (Cole et al., 1993; Wedaman et al., 1996). Such plus-end-directed motility would correspond to anterograde movement from the base to the tip of cilia. By microinjecting monoclonal antibodies into fertilized sea urchin eggs to inhibit the function of heterotrimeric kinesin-2 and monitoring subsequent embryonic development, we observed no effect on mitosis but we did observe a dramatic inhibition of motile cilia assembly on the blastula-stage embryo, suggesting that heterotrimeric kinesin-2 is required for ciliogenesis (Morris and Scholey, 1997).
How kinesin-2 motors might contribute to ciliogenesis was illuminated by pioneering studies done in parallel in Chlamydomonas flagella, leading to the discovery of intraflagellar transport (IFT), the bidirectional, kinesin-2 and IFT-dynein dependent transport of multi-subunit macromolecular complexes called IFT-particles along axonemal MTs between the base and the tip of the axoneme which is essential for cilium assembly and maintenance (Cole et al., 1998; Kozminski et al., 1993; Rosenbaum and Witman, 2002). Early on it was recognized that IFT might contribute to ciliogenesis in a broad range of systems, including vertebrate photoreceptors (Rosenbaum et al., 1999). Evidence in support of this hypothesis is discussed later (section 5).
3. Studying kinesin-2 motors, IFT and ciliogenesis in the C. elegans Nervous System; two anterograde IFT motors cooperate to move IFT particles along the Axoneme
To improve our understanding of how kinesin-2 motors contribute to IFT and ciliogenesis, we decided to turn to the nematode, Caenorhabditis elegans which Sydney Brenner had introduced as a model system for studying the genetics of the nervous system (Brenner, 1974; White et al., 1986). Advantages included the existence of multiple behavioural mutants with defective cilia on their sensory neurons, which might be useful for dissecting mechanisms of ciliogenesis (Perkins et al., 1986; Starich et al., 1995) and techniques for using green fluorescent protein (GFP) to monitor the expression and dynamics of neuronal proteins (Chalfie et al., 1994). Also, when we first sequenced the novel KAP subunit of sea urchin heterotrimeric kinesin-2, the only homologous sequence we found was located on a cosmid present in the C. elegans genome sequence database which we proposed might encode a KAP that oligomerizes with OSM-3 (Tabish et al., 1995) plus a second motor subunit to form a single heterotrimeric kinesin-2 motor complex that would be amenable to functional analysis via mutants (Wedaman et al., 1996).
To our surprise, using biochemical fractionation and molecular biology, we found that C. elegans sensory cilia contain two kinesin-2 holoenzymes, a heterotrimeric form, kinesin-II, much like the prototypic sea urchin motor, and a homodimeric kinesin-2 termed “OSM-3” (Signor et al., 1999) (Fig. 2). This was supported by the subsequent expression, purification and biochemical characterization of recombinant C. elegans kinesin-II and OSM-3 (Imanishi et al., 2006; Pan et al., 2006).
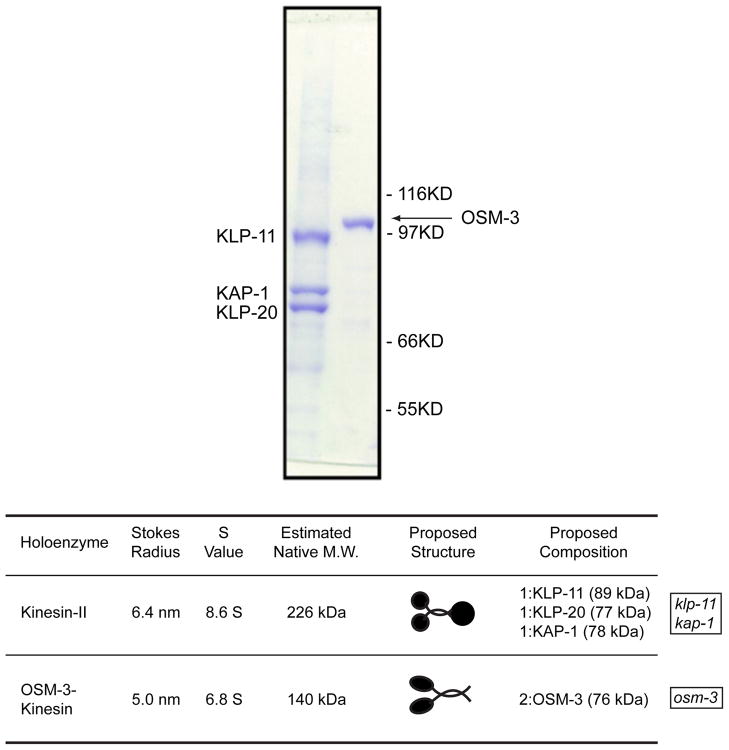
Figure 2.
C. elegans sensory cilia contain two kinesin-2 holoenzymes, heterotrimeric kinesin-II and homodimeric OSM-3. Upper panel, coomassie stained SDS PAGE of purified recombinant kinesin-II and GFP-tagged OSM-3 (Pan et al., 2006). Lower panel, oligomeric state of native kinesin-II and OSM-3 determined by biochemical fractionation of C. elegans extracts Signor et al., 1999). Kinesin-II mutants (klp-11 and kap-1) and osm-3 mutants used in Fig. 3 are indicated.
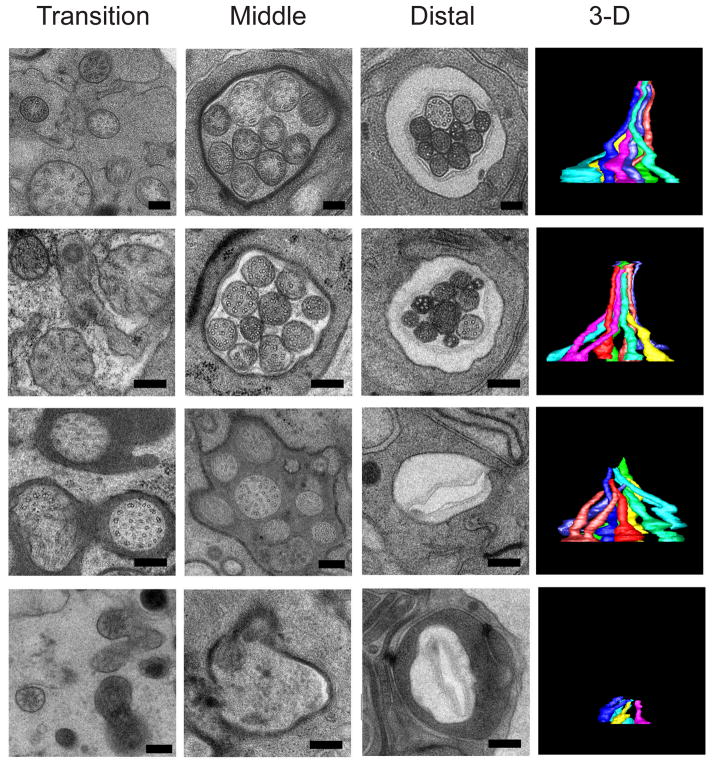
To understand if and how kinesin-II and OSM-3 contribute to axoneme assembly, we compared the structure of sensory cilia by light microscopy and serial section EM with 3D reconstructions in wild type versus single mutants lacking kinesin-II or OSM-3 function, and double mutants lacking both kinesin-II and OSM-3 function (Fig. 3) (Evans et al., 2006; Snow et al., 2004). Ciliary axonemes in wild types and kinesin-II mutants contain intact middle and distal segments (Fig. 3, upper two rows), those in osm-3 mutants specifically lack distal segments (Fig. 3, 3rd row and (Perkins et al., 1986)) whereas double kinesin-II;osm-3 mutants lack the entire axoneme (Fig. 3 bottom row). Thus kinesin-II and OSM-3 motors function redundantly to assemble the middle segment, i.e. the axoneme core, whereas OSM-3 alone specifically extends the distal singlets.
Figure 3.
Serial section EM through the transition zone, middle segment and distal segment, and 3D reconstructions showing a bundle of amphid channel cilia in (top row) wild type animals, (second row) mutant animals lacking kinesin-II function, (third row) mutant animals lacking OSM-3 function, and (bottom row) double mutants lacking both OSM-3 and kinesin-II function (Evans et al., 2006).
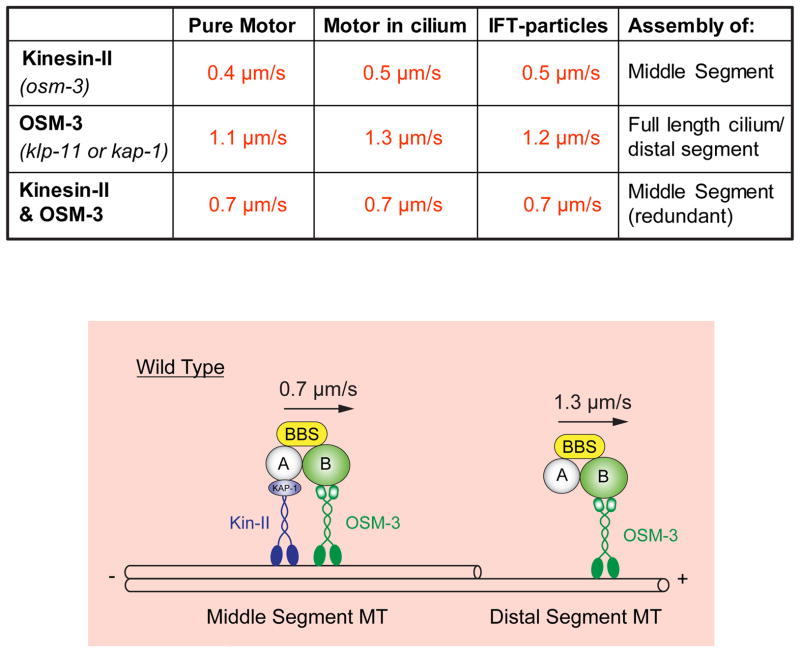
To evaluate the role of IFT driven by kinesin-2 motors in ciliogenesis, we developed time lapse fluorescence microscopy assays to measure the rates of movement of specifically labeled IFT proteins along sensory cilia (Orozco et al., 1999). Measurements of the rates of movement of tagged IFT-particle subunits, kinesin-II and OSM-3 suggested that kinesin-II alone moves IFT particles at 0.4–0.5μm/s, OSM-3 alone moves IFT particles at 1.1–1.3μm/s, and the two motors can work together to move the same IFT particles at 0.7μm/s (Fig. 4, Table) (Ou et al., 2005; Snow et al., 2004) and it was possible to replicate these rates using purified kinesin-II and OSM-3 in motility assays in which the rate of MT gliding was measured as a function of the mole fraction of mixtures of the two motors (Fig 4, Table) (Pan et al., 2006). This has led to the model in which, in wild type cilia, kinesin-II and OSM-3 move IFT particles from the basal body to the tip of the middle segment at the intermediate rate of 0.7μm/s to build the middle segment, at the tip of the middle segment kinesin-II dissociates, possibly in response to MAP kinase activity (Burghoorn et al., 2007), and then OSM-3 alone moves IFT particles the rest of the way along the distal singlets to specifically assemble the distal segment (Fig. 4, lower panel).
Figure 4.
Model for IFT along C. elegans sensory cilia inferred from in vitro motility and in vivo transport assays. Upper table shows rates of MT gliding driven by purified recombinant kinesin-II, OSM-3, or mixtures of kinesin-II and OSM-3 that reconstitute the rates of transport of IFT-particles along cilia driven by kinesin-II alone (in osm-3 or bbs mutants), OSM-3 alone (in kinesin-II or bbs mutants) or kinesin-II/OSM-3 together (along wild type middle segments). Lower panel, cartoon model showing how kinesin-II and OSM-3 together move IFT particles along the cilium middle segments to build the middle segments, then OSM-3 alone moves IFT-particles along the distal segments to assemble the distal segments. See (Ou et al., 2005; Pan et al., 2006; Snow et al., 2004).
While this model is consistent with available data for amphid channel and phasmid sensory cilia, there is evidence that these two motors are deployed differently in other classes of C. elegans sensory cilia e.g. (Evans et al., 2006; Morsci and Barr, 2011; Mukhopadhyay et al., 2007). This underscores the complexity in the mechanisms by which different kinesin motors are utilized in different types of cilia, even in the same organism, and has led to the hypothesis that “core” heterotrimeric kinesin-2 motors which build the axoneme core, can be modulated in various ways by “accessory” motors e.g. OSM-3, to confer cilia-specific properties such as the extension of distal singlet MTs (Scholey, 2008).
4. Dissecting the distal singlet pathway in C. elegans; ciliary tubulin isotypes as potential cargo
We initially focused our attention on distal singlet MTs based on the idea that they might serve as specialized signaling domains in sensory cilia (introduction). We reasoned that components of the distal segment assembly pathway might include cargo molecules that are transported by the IFT machinery to the distal tips of the ciliary axonemes where they incorporate into the singlets. To identify such components, we analyzed all the ciliary (che, osm, dyf) mutants for those that phenocopy the osm-3 mutant in specifically missing the distal singlets of their cilia and we sequenced some of their products (Hao et al., 2011b; Ou et al., 2005; Ou et al., 2007).
One class of proteins identified in this screen included IFT-particle subcomplex B subunits that are required for OSM-3 driven transport along the cilium, including DYF-1, DYF-6 and IFT-74/81(Hao et al., 2011b; Ou et al., 2005). A second class included subunits of the BBSome (Nachury et al., 2007) which had previously been shown to undergo IFT (Blacque et al., 2004) and were required to maintain the integrity of IFT-particle subcomplexes A and B (Ou et al., 2005; Ou et al., 2007; Pan et al., 2006). Used in concert with transport assays using specifically tagged markers, these latter mutants were very useful for assigning C. elegans proteins as subunits of either IFT-A or IFT-B (Ou et al., 2007). Finally, another class of distal segment mutants identified the α and β tubulin isotypes, TBA-5 and TBB-4 as potential cargo of IFT in these cilia.
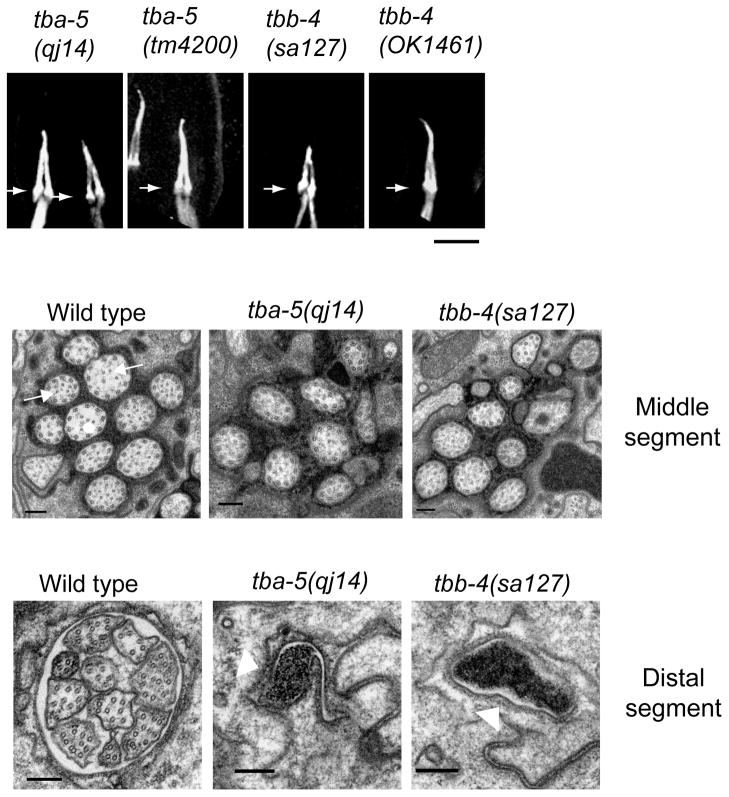
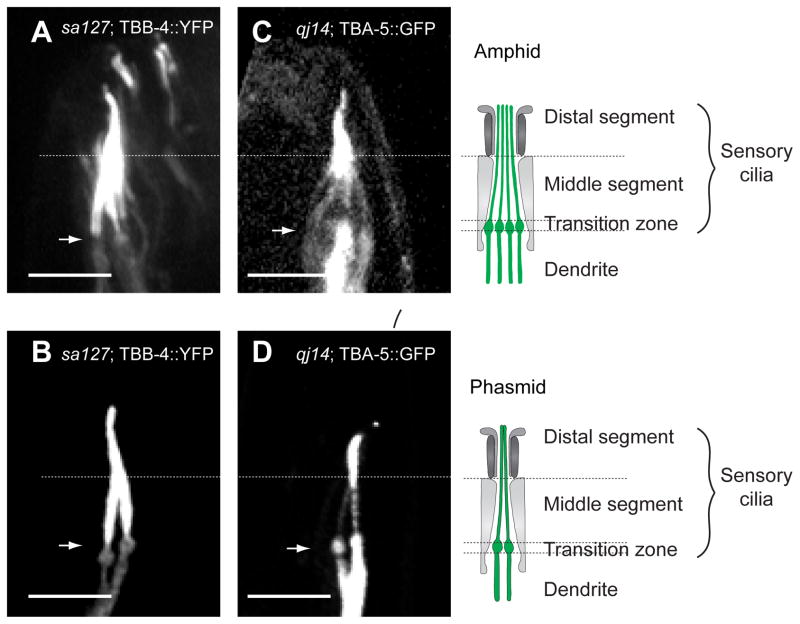
We found that specific point mutations, A19V and P360L in TBA-5 and L253F in TBB-4 specifically destabilize singlet MTs, whereas deletion mutants do not (Fig. 5). The point mutations may therefore be categorized as “recessive, gain of function” mutations (Wright and Hunter, 2003). The results suggest that these tubulins are not essential for ciliary axoneme assembly and that compensatory tubulins (Hurd et al., 2010) can functionally substitute for the deletion of TBA-4 and TBA-5, whereas specific point mutants within these tubulins can “poison” singlet MT stability (Hao et al., 2011b). Moreover these tubulins are differentially localized, with TBB4 being found all along the axoneme and TBA-5 being concentrated in the distal singlets (Fig. 6) suggesting a possible differentiation of function.
Figure 5.
Missense mutations in the ciliary α-tubulin TBA-5 and β-tubulin TBB-4 specifically destabilize sensory cilium distal singlets whereas deletion mutants do not. Upper row, light micrographs of cilia in strains expressing the destabilizing missense mutants (qj14, sa127) and the deletion mutants (tm4200, OK1461). Lower rows, EM of sections through the middle and distal segments of cilia in wild types, tbb-4 and tba-5 missense mutant animals confirming the loss of distal segment (and in tbb-4, central middle segment) singlet MTs (Hao et al., 2011b).
Figure 6.
Tubulins TBB-4 and TBA-5 are differentially localized within the sensory cilia axonemes. Fluorescent TBB-4 is concentrated all along the axoneme in amphid (A) and phasmid (B) cilia, whereas TBA-5 is concentrated in the distal segments (C, D) (and is also expressed in dendrites) (Hao et al., 2011b).
When we used our standard fluorescence microscopy/kymography IFT assays to determine if these tubulins are delivered by IFT, only very faint diagonal tracks were visible (Hao et al., 2011b). Two other potential cargo molecules of kinesin-2 motors have been identified using these types of IFT assays; subunits of the retrograde motor, IFT-dynein, which yield very robust tracks in kymographs (but appear to move independent of IFT-particles) (Hao et al., 2011a) and ciliary membrane associated TRPV channels which yield less robust tracks (Qin, 2005).
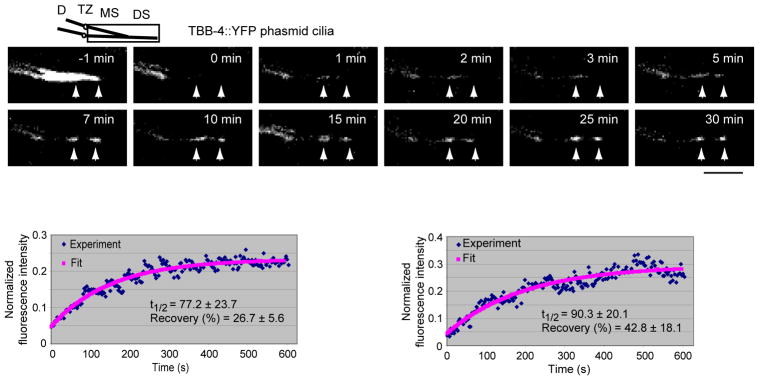
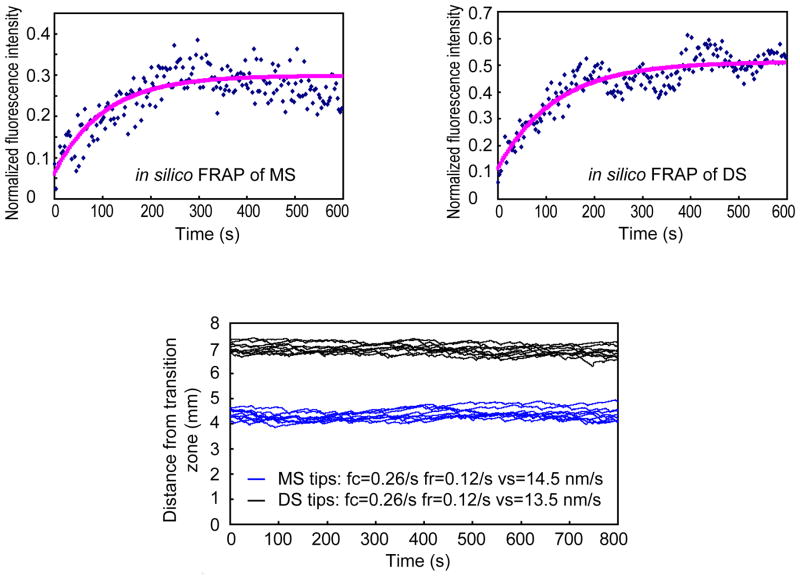
Because the kymographs of moving fluorescent TBB-4 were faint and not totally convincing, we analyzed the dynamics of the protein using FRAP and mathematical modeling (Hao et al., 2011b). We found that, following photobleaching, new fluorescent TBB-4 assembled at the tips of the middle and distal segments with a FRAP half-time of the order of 1–2 minutes (Fig. 7). Modeling revealed that the dynamics of the observed recovery could be accounted for by IFT of the tubulin subunits to the MT tips at rates driven by the cooperative action of kinesin-II and OSM-3 along wild type cilia, followed by insertion at the tips by dynamic instability, but substituting diffusion or rates of IFT corresponding to mutants lacking either kinesin-II or OSM-3 function did not give a good fit to the data (Fig. 8) (Hao et al., 2011b). This may explain why this elaborate two-motor anterograde IFT pathway is used – namely to optimize the speed of tubulin transport for optimum cilium length, in accordance with the balance point model for cilium length control (Engel et al., 2009; Hao et al., 2011b; Marshall and Rosenbaum, 2001). The model also predicts that 2 or 3 tubulins are delivered per IFT particle, but in kymographs we see only faint tracks because fluorescent TBB-4 on moving IFT particles is “diluted out” by exchange with; (i) non-fluorescent TBB-4 subunits created by the photobleaching that is needed to reduce the high background fluorescence of already incorporated axonemal tubulins; and (ii) by other ciliary tubulin isotypes (Hao et al., 2011b). It is possible, however, that standard IFT assays of more intensely labeled fluorescent tubulins (e.g. by adding multiple tags) could yield more robust tracks in kymographs. Further work on the delivery of ciliary tubulins by IFT and by other complementary mechanisms would be beneficial.
Figure 7.
Fluorescence Recovery After Photobleaching (FRAP) analysis of tubulin, TBB-4 dynamics in phasmid sensory cilia. Upper panels, the entire ciliary axonemes of phasmids expressing fluorescent TBB-4 (−1 min) was photobleached (0 min) and its recovery by incorporation of fluorescent tubulin at the tips of the middle and distal segment MTs (arrowheads) was monitored over the subsequent 30 minutes. Lower panels, FRAP recovery curves for middle (left) and distal (right) segments (Hao et al., 2011b).
Figure 8.
Results of mathematical modeling of the ciliary tubulin FRAP data. The model, which was derived from the “balance point” model (Engel et al., 2009; Marshall et al., 2005; Marshall and Rosenbaum, 2001), yields results consistent with the idea that the delivery of tubulin subunits by IFT at rates corresponding to those thought to be mediated by kinesin-II and OSM-3 in wild type cilia (Fig. 4) can maintain a cilium at its steady state length, can account for the FRAP recovery curves (upper) and can maintain axonemal MT tips in close axial proximity while they incorporate tubulin subunits at their plus ends via dynamic instability (lower). In contrast, substituting diffusion or rates of IFT driven by kinesin-II alone or OSM-3 alone as in mutant cilia does not yield a good fit to experimental data (Hao et al., 2011b).
5. Kinesin-2 motors and IFT in Vertebrate Photoreceptor Cilia
Work done mainly in zebrafish and rodents suggests that, as in C. elegans sensory cilia, members of the kinesin-2 family play important roles in photoreceptor cilium assembly and maintenance, but the mechanism by which they do so is currently less clear.
Observations that mammalian photoreceptor OS turnover approximately 10% of their length per day suggest that OS membrane proteins such as rhodopsin must be rapidly transported into the OS, whereas light stimulates the translocation of 109 arrestin molecules per hour from the IS to the OS, consistent with a rate of trafficking several hundred-fold faster than rhodopsin (Insinna and Besharse, 2008). Current data suggest that rhodopsin trafficking depends on the kinesin-2 family motors, heterotrimeric kinesin-II (composed of KIF3A, KIF3B or KIF3C plus KAP3) and homodimeric kinesin-2 (composed of KIF17) plausibly acting via IFT and complemented by the actin-based motor, myosin VIIa (Williams, 2002). In contrast, arrestin transport appears too fast for a cytoskeletal motor-dependent translocation and is proposed to depend on some type of rapid diffusion-to-capture involving capture by photoactivated OS binding sites (Calvert et al., 2006; Insinna and Besharse, 2008).
In rodents, loss of heterotrimeric kinesin-II function by the targeted mutagenesis of KIF3A and loss of function of the IFT particle subunit, IFT-88 led to abnormal OS morphogenesis, rhodopsin mislocalization to the IS and retinal degeneration characterized by a progressive loss of photoreceptors. This supports the hypothesis that kinesin-II-driven IFT may transport rhodopsin and other membrane-bound OS components from the IS, through the connecting cilium and out along the axoneme to assemble and maintain the OS (Jimeno et al., 2006; Lopes et al., 2010; Marszalek et al., 2000; Pazour et al., 2002). A role for kinesin-II in such trafficking is supported by the expression of dominant negative kinesin-II in zebrafish cone photoreceptors which disrupted the IS and synaptic ribbon leading to cell death (Insinna et al., 2009). However, the situation is complicated by complexity in the manner of deployment of heterotrimeric kinesin-II motors; in zebrafish, the analysis of loss-of-function KIF3B single versus KIF3B/3C double mutants has led to proposals that early photoreceptor morphogenesis depends only on KIF3A/3B/KAP3 whereas later development depends on either KIF3A/3B/KAP3 or KIF3A/3C/KAP3 functioning in a redundant manner (Zhao et al., 2012). Moreover, targeted knock-out of KIF3A in specific types of rodent photoreceptors led to proposals that heterotrimeric kinesin-II trafficks membrane proteins to cone but not rod outer segments (Avasthi et al., 2009). This challenges the appealing hypothesis that the mislocalization of opsin to the IS observed in rod photoreceptors of kinesin-II knock-out mice reflects defects in specific kinesin-II-driven transport of rhodopsin to the OS and further work on this important topic is required (Avasthi et al., 2009; Lopes et al., 2010).
The role of the homodimeric kinesin-2 motor, KIF17, in vertebrate photoreceptor ciliogenesis is also an active area of investigation. In one study, loss-of-function mutations in zebrafish KIF17 were associated with only slight truncations in the distal singlets of olfactory cilia whereas the gross morphology of photoreceptors appeared perfectly normal, although it remains to be determined if the mutation eliminates all KIF17 function in these cells, or if minor defects in the trafficking of membrane proteins to the OS was overlooked (Zhao et al., 2012). In contrast, the morpholino-based depletion of KIF17 led to a delay in photoreceptor morphogenesis and dominant negative KIF17 constructs specifically disrupted the OS, perhaps because KIF17 functions by specifically elongating the photoreceptor axonemal singlet MTs and/or by delivering membrane proteins to the OS (Insinna et al., 2009). A role in distal singlet assembly is consistent with the role of OSM-3 in C. elegans, whereas the latter is consistent with the idea that KIF17 delivers cyclic nucleotide gated channels to the distal tip of some vertebrate primary cilia (Verhey et al., 2011).
Thus there are strong suggestions that both heterotrimeric kinesin-2 and homodimeric kinesin-2 motors contribute to the morphogenesis of vertebrate photoreceptor cilia, but whether they do so by driving IFT as seems highly plausible, and if they act in a semi-redundant fashion to drive sequential steps of anterograde IFT as proposed for C elegans amphid channel and phasmid sensory cilia (Fig. 3, 4) requires further work.
Conclusions
As noted in the introduction, in some ways the sensory outer segments of vertebrate photoreceptor cilia are equivalent to the distal segments of C. elegans sensory cilia on the dendritic endings of amphid and phasmid chemosensory neurons, and thus the experimentally more pliable C. elegans sensory cilia may represent useful models for studying the role of kinesin-2 motors and IFT in the medically more relevant vertebrate photoreceptor cilia (see also (Mok and Heon, 2012)).
The available evidence reviewed above is consistent with the hypothesis that kinesin-2 motors contribute to the assembly, maintenance and function of both C. elegans sensory cilia and vertebrate photoreceptor cilia. In amphid and phasmid channel C. elegans cilia, this evidence supports the hypothesis that heterotrimeric kinesin-II and homodimeric OSM-3 function redundantly to assemble the axoneme core (aka middle segments) whereas OSM-3 alone extends the distal singlets, creating a two-step pathway of anterograde transport that delivers IFT particles, retrograde dynein motors and ciliary tubulin subunits to tips of the axoneme and also moves TRPV channels within the plane of the ciliary membrane, although it is quite possible that future work will necessitate revisions of this model. There is also evidence that the heterotrimeric kinesin-II/KIF3 and homodimeric KF17 motors play important roles in photoreceptor morphogenesis. At this stage there exists good evidence that kinesin-II is required for delivery of opsins and possibly other membrane-bound cargo to the OS, especially in cones, and although a role for IFT in this process seems likely, direct evidence is lacking. Roles for KIF17 in OS morphogenesis is supported by some, but not all published work, and whether KIF17 cooperates with kinesin-II in a pathway similar to that seen in C. elegans cilia is unclear, though work addressing this issue is progressing very well.
Dissecting the mechanisms by which kinesin-2 motors cooperate in the delivery of specific cargoes to the sensory OS of vertebrate photoreceptors and evaluating the roles of IFT versus other mechanisms such as diffusion and actin-based transport would benefit greatly from the development of; (a) time-lapse fluorescence microscopy and kymography-based assays of the type first developed in C. elegans sensory cilia; and (b) the combined use of FRAP and mathematical modeling of the type used to evaluate role of IFT in tubulin dynamics in these cilia. Although this is technically far more challenging in vertebrate photoreceptor cilia, evidence that excellent progress is being made was reported at this meeting. In my opinion, it is through the application of such technical approaches, rather than the application of specific models that work on C. elegans sensory ciliogenesis can best aid in understanding how kinesin-2 motors, IFT and other transport pathways contribute to vertebrate photoreceptor OS assembly and function, and how trafficking defects give rise to photoreceptor degeneration and visual impairment.
Acknowledgments
Many thanks to Drs Wolfgang Baer and Eric Pierce for the invitation to present this talk at the excellent 14th Vision Research Conference on Retina Ciliopathies, and to the many lab members and collaborators who contributed to the research. I thank Dr David Williams and two anonymous referees for comments. This project received over two decades of generous funding from the NIH (GM 50718), American Cancer Society and March of Dimes Birth Defects Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avasthi P, Watt CB, Williams DS, Le YZ, Li S, Chen CK, Marc RE, Frederick JM, Baehr W. Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric kinesin-II. J Neurosci. 2009;29:14287–14298. doi: 10.1523/JNEUROSCI.3976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Mori I. Chemotaxis and Thermotaxis. In: Riddle Donald L, TB, Meyer Barbara J, Priess James R., editors. C. elegans II. Cold Spring Harbor Laboratory Press; 1997. pp. 717–737. [PubMed] [Google Scholar]
- Blacque OE, Reardon MJ, Li C, McCarthy J, Mahjoub MR, Ansley SJ, Badano JL, Mah AK, Beales PL, Davidson WS, Johnsen RC, Audeh M, Plasterk RH, Baillie DL, Katsanis N, Quarmby LM, Wicks SR, Leroux MR. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 2004;18:1630–1642. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghoorn J, Dekkers MP, Rademakers S, de Jong T, Willemsen R, Jansen G. Mutation of the MAP kinase DYF-5 affects docking and undocking of kinesin-2 motors and reduces their speed in the cilia of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:7157–7162. doi: 10.1073/pnas.0606974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends in cell biology. 2006;16:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Cole DG, Chinn SW, Wedaman KP, Hall K, Vuong T, Scholey JM. Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature. 1993;366:268–270. doi: 10.1038/366268a0. [DOI] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel BD, Ludington WB, Marshall WF. Intraflagellar transport particle size scales inversely with flagellar length: revisiting the balance-point length control model. J Cell Biol. 2009;187:81–89. doi: 10.1083/jcb.200812084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JE, Snow JJ, Gunnarson AL, Ou G, Stahlberg H, McDonald KL, Scholey JM. Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J Cell Biol. 2006;172:663–669. doi: 10.1083/jcb.200509115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Efimenko E, Swoboda P, Scholey JM. The retrograde IFT machinery of C. elegans cilia: two IFT dynein complexes? PloS one. 2011a;6:e20995. doi: 10.1371/journal.pone.0020995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Thein M, Brust-Mascher I, Civelekoglu-Scholey G, Lu Y, Acar S, Prevo B, Shaham S, Scholey JM. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat Cell Biol. 2011b;13:790–798. doi: 10.1038/ncb2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd DD, Miller RM, Nunez L, Portman DS. Specific alpha- and beta-tubulin isotypes optimize the functions of sensory Cilia in Caenorhabditis elegans. Genetics. 2010;185:883–896. doi: 10.1534/genetics.110.116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi M, Endres NF, Gennerich A, Vale RD. Autoinhibition regulates the motility of the C. elegans intraflagellar transport motor OSM-3. J Cell Biol. 2006;174:931–937. doi: 10.1083/jcb.200605179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis PN, Ou G, Leroux MR, Scholey JM. WormBook: the online review of C. elegans biology. 2007. The sensory cilia of Caenorhabditis elegans; pp. 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237:1982–1992. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Humby M, Sedmak T, Wolfrum U, Besharse JC. Different roles for KIF17 and kinesin II in photoreceptor development and maintenance. Dev Dyn. 2009;238:2211–2222. doi: 10.1002/dvdy.21956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- Jimeno D, Feiner L, Lillo C, Teofilo K, Goldstein LS, Pierce EA, Williams DS. Analysis of kinesin-2 function in photoreceptor cells using synchronous Cre-loxP knockout of Kif3a with RHO-Cre. Investigative ophthalmology & visual science. 2006;47:5039–5046. doi: 10.1167/iovs.06-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, Goldstein LS, Goodson HV, Hirokawa N, Howard J, Malmberg RL, McIntosh JR, Miki H, Mitchison TJ, Okada Y, Reddy AS, Saxton WM, Schliwa M, Scholey JM, Vale RD, Walczak CE, Wordeman L. A standardized kinesin nomenclature. J Cell Biol. 2004;167:19–22. doi: 10.1083/jcb.200408113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes VS, Jimeno D, Khanobdee K, Song X, Chen B, Nusinowitz S, Williams DS. Dysfunction of heterotrimeric kinesin-2 in rod photoreceptor cells and the role of opsin mislocalization in rapid cell death. Mol Biol Cell. 2010;21:4076–4088. doi: 10.1091/mbc.E10-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF, Qin H, Rodrigo Brenni M, Rosenbaum JL. Flagellar length control system: testing a simple model based on intraflagellar transport and turnover. Mol Biol Cell. 2005;16:270–278. doi: 10.1091/mbc.E04-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF, Rosenbaum JL. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol. 2001;155:405–414. doi: 10.1083/jcb.200106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- Mok CA, Heon E. Caenorhabditis elegans as a model organism for ciliopathies and related forms of photoreceptor degeneration. Advances in experimental medicine and biology. 2012;723:533–538. doi: 10.1007/978-1-4614-0631-0_67. [DOI] [PubMed] [Google Scholar]
- Morris RL, Scholey JM. Heterotrimeric kinesin-II is required for the assembly of motile 9+2 ciliary axonemes on sea urchin embryos. J Cell Biol. 1997;138:1009–1022. doi: 10.1083/jcb.138.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsci NS, Barr MM. Kinesin-3 KLP-6 regulates intraflagellar transport in male-specific cilia of Caenorhabditis elegans. Curr Biol. 2011;21:1239–1244. doi: 10.1016/j.cub.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Lu Y, Qin H, Lanjuin A, Shaham S, Sengupta P. Distinct IFT mechanisms contribute to the generation of ciliary structural diversity in C. elegans. The EMBO journal. 2007;26:2966–2980. doi: 10.1038/sj.emboj.7601717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Orozco JT, Wedaman KP, Signor D, Brown H, Rose L, Scholey JM. Movement of motor and cargo along cilia. Nature. 1999;398:674. doi: 10.1038/19448. [DOI] [PubMed] [Google Scholar]
- Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–587. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- Ou G, Koga M, Blacque OE, Murayama T, Ohshima Y, Schafer JC, Li C, Yoder BK, Leroux MR, Scholey JM. Sensory ciliogenesis in Caenorhabditis elegans: assignment of IFT components into distinct modules based on transport and phenotypic profiles. Mol Biol Cell. 2007;18:1554–1569. doi: 10.1091/mbc.E06-09-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Ou G, Civelekoglu-Scholey G, Blacque OE, Endres NF, Tao L, Mogilner A, Leroux MR, Vale RD, Scholey JM. Mechanism of transport of IFT particles in C. elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J Cell Biol. 2006;174:1035–1045. doi: 10.1083/jcb.200606003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Qin H, Burnette, Bae Y-K, Forscher P, Barr MM, Rosenbaum JL. IFT is required for the vectorial movement of TRPV channels in the ciliary membrane. Current Biology. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Cole DG, Diener DR. Intraflagellar transport: the eyes have it. J Cell Biol. 1999;144:385–388. doi: 10.1083/jcb.144.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Scholey JM. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J Cell Biol. 2008;180:23–29. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signor D, Wedaman KP, Rose LS, Scholey JM. Two heteromeric kinesin complexes in chemosensory neurons and sensory cilia of Caenorhabditis elegans. Mol Biol Cell. 1999;10:345–360. doi: 10.1091/mbc.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–1113. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- Starich TA, Herman RK, Kari CK, Yeh WH, Schackwitz WS, Schuyler MW, Collet J, Thomas JH, Riddle DL. Mutations affecting the chemosensory neurons of Caenorhabditis elegans. Genetics. 1995;139:171–188. doi: 10.1093/genetics/139.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabish M, Siddiqui ZK, Nishikawa K, Siddiqui SS. Exclusive expression of C. elegans osm-3 kinesin gene in chemosensory neurons open to the external environment. J Mol Biol. 1995;247:377–389. doi: 10.1006/jmbi.1994.0146. [DOI] [PubMed] [Google Scholar]
- Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey KJ, Dishinger J, Kee HL. Kinesin motors and primary cilia. Biochem Soc Trans. 2011;39:1120–1125. doi: 10.1042/BST0391120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedaman KP, Meyer DW, Rashid DJ, Cole DG, Scholey JM. Sequence and submolecular localization of the 115-kD accessory subunit of the heterotrimeric kinesin-II (KRP85/95) complex. J Cell Biol. 1996;132:371–380. doi: 10.1083/jcb.132.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Williams DS. Transport to the photoreceptor outer segment by myosin VIIa and kinesin II. Vision research. 2002;42:455–462. doi: 10.1016/s0042-6989(01)00228-0. [DOI] [PubMed] [Google Scholar]
- Wright AJ, Hunter CP. Mutations in a beta-tubulin disrupt spindle orientation and microtubule dynamics in the early Caenorhabditis elegans embryo. Mol Biol Cell. 2003;14:4512–4525. doi: 10.1091/mbc.E03-01-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139:246–264. doi: 10.1016/j.cell.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Omori Y, Brodowska K, Kovach P, Malicki J. Kinesin-2 family in vertebrate ciliogenesis. Proc Natl Acad Sci U S A. 2012;109:2388–2393. doi: 10.1073/pnas.1116035109. [DOI] [PMC free article] [PubMed] [Google Scholar]