Abstract
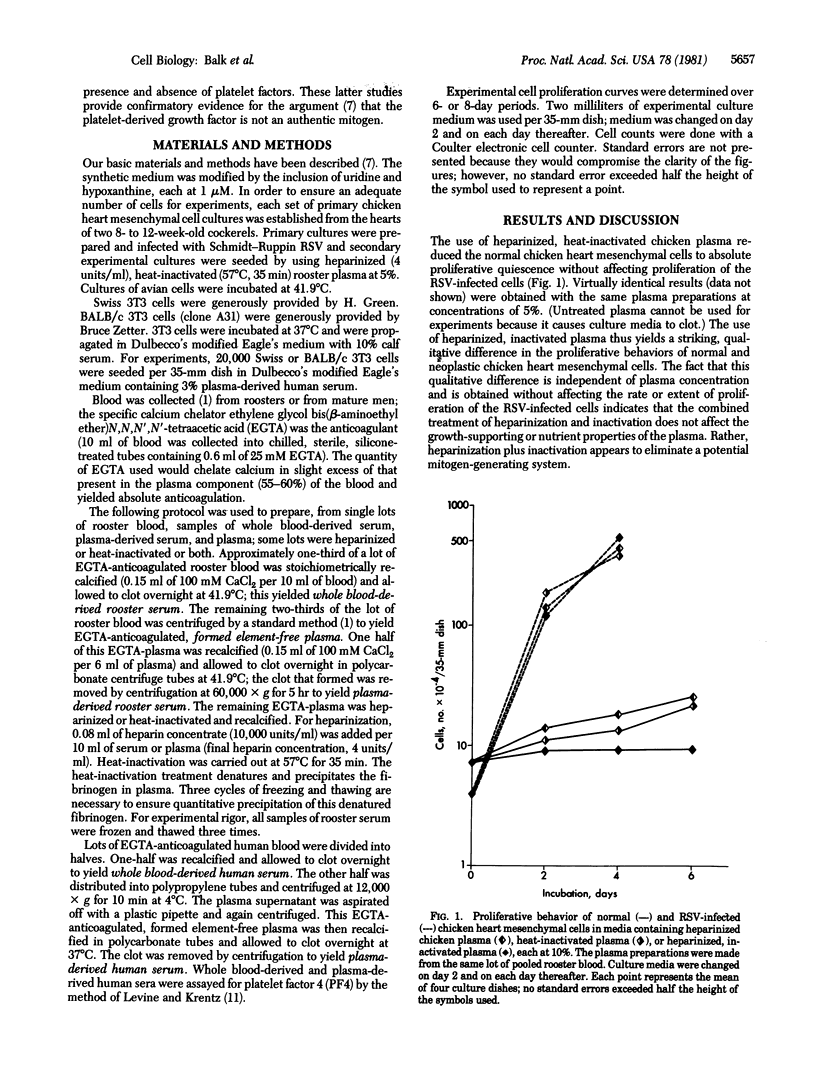
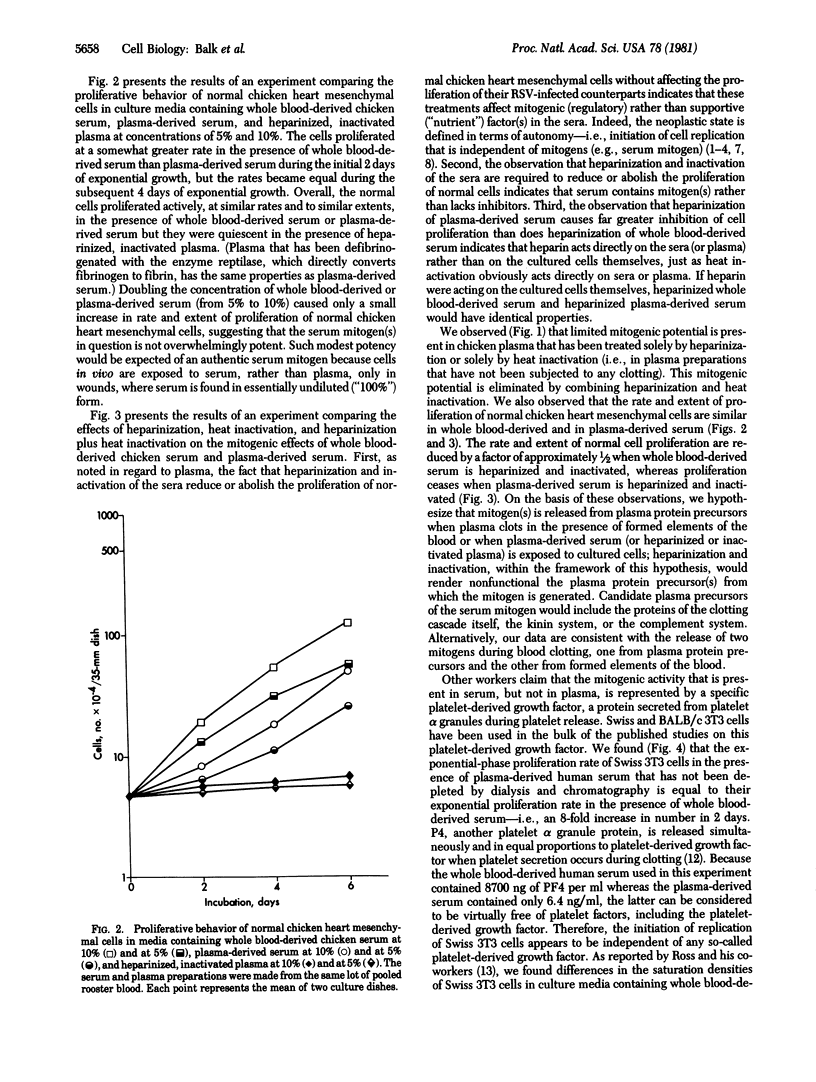
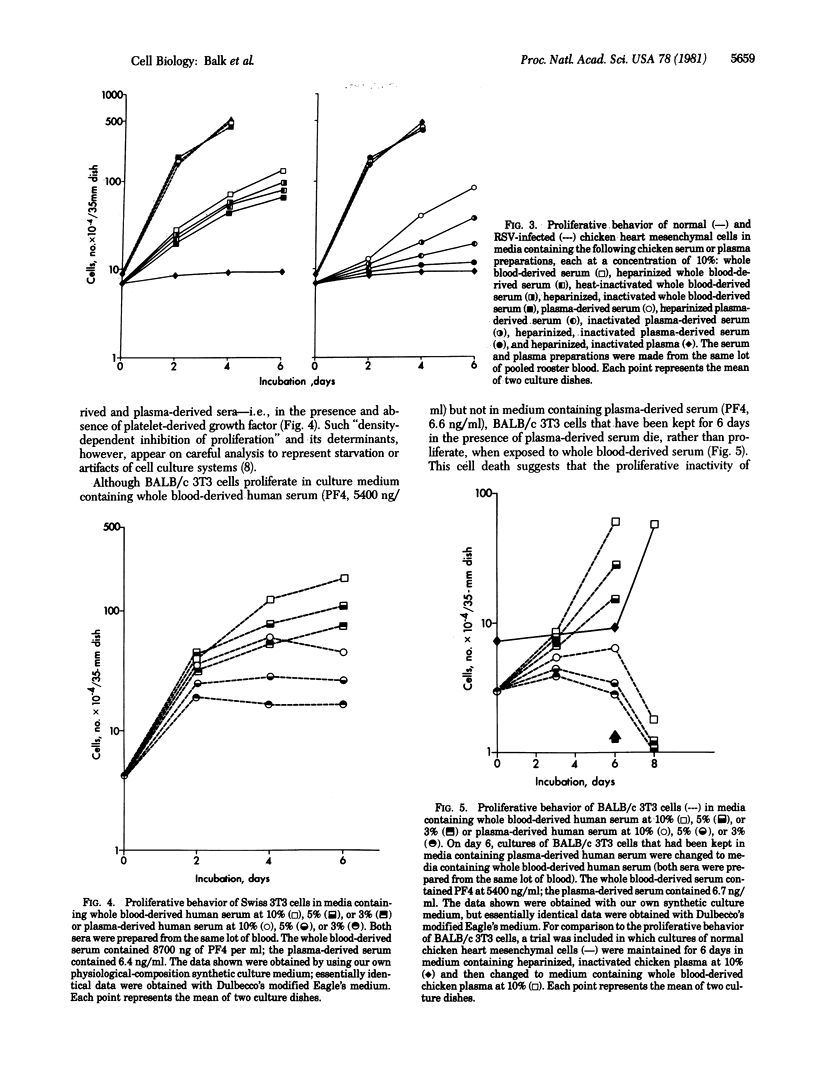
In culture medium containing heparinized, heat-inactivated, chicken plasma, normal chicken heart mesenchymal cells do not proliferate but their Rous sarcoma virus-infected counterparts proliferate maximally. In medium containing serum derived from chicken whole blood or plasma, on the other hand, normal chicken heart mesenchymal cells proliferate actively, at similar overall rates and to similar extents. The rate and extent of normal cell proliferation are decreased by a factor of approximately 1/2 with whole blood-derived serum that is heparinized and inactivated; proliferation ceases in plasma-derived serum that is heparinized and inactivated. Heparinization and inactivation of serum does not affect the proliferation of Rous sarcoma virus-infected cells, indicating that this combined treatment eliminates a mitogenic (regulatory) rather than a supportive (nutrient) factor(s) for cell replication. We hypothesize that mitogen(s) is released from plasma protein precursors when plasma clots in the presence of formed elements of the blood or when plasma-derived serum is exposed to cultured cells; heparinization and inactivation, within the framework of this hypothesis, would render nonfunctional the plasma protein precursor(s) from which the mitogen(s) is generated. Alternatively, our data are consistent with the release of two mitogens during blood clotting, one from plasma protein precursors and the other from formed elements of the blood. We also have studied the proliferative behavior of Swiss and BALB/c 3T3 cells in whole blood-derived and plasma-derived human serum. Our studies suggest that the platelet-derived growth factor has an artifactual supportive (nutrient) role, rather than an authentic mitogenic role, in cell replication.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azizkhan J. C., Khagsbrun M. Chondrocytes contain a growth factor that is localized in the nucleus and is associated with chromatin. Proc Natl Acad Sci U S A. 1980 May;77(5):2762–2766. doi: 10.1073/pnas.77.5.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D. Active proliferation of Rous sarcoma virus-infected, but not normal, chicken heart mesenchymal cells in culture medium of physiological composition. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6606–6610. doi: 10.1073/pnas.77.11.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D. Calcium as a regulator of the proliferation of normal, but not of transformed, chicken fibroblasts in a plasma-containing medium. Proc Natl Acad Sci U S A. 1971 Feb;68(2):271–275. doi: 10.1073/pnas.68.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D., Polimeni P. I., Hoon B. S., LeStourgeon D. N., Mitchell R. S. Proliferation of Rous sarcoma virus-infected, but not of normal, chicken fibroblasts in a medium of reduced calcium and magnesium concentration. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3913–3916. doi: 10.1073/pnas.76.8.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D. Precipitates and particulates cause proliferative activity of density-inhibited cultured cells by disturbing the diffusion boundary layer: an artefact superimposed upon an artefact? Life Sci. 1980 Nov 24;27(21):1917–1920. doi: 10.1016/0024-3205(80)90409-9. [DOI] [PubMed] [Google Scholar]
- Balk S. D. Stimulation of the proliferation of chicken fibroblasts by folic acid or a serum factor(s) in a plasma-containing medium. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1689–1692. doi: 10.1073/pnas.68.8.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D., Whitfield J. F., Youdale T., Braun A. C. Roles of calcium, serum, plasma, and folic acid in the control of proliferation of normal and Rous sarcoma virus-infected chicken fibroblasts. Proc Natl Acad Sci U S A. 1973 Mar;70(3):675–679. doi: 10.1073/pnas.70.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone C. W. Malignant hemangioendotheliomas produced by subcutaneous inoculation of Balb/3T3 cells attached to glass beads. Science. 1975 Apr 4;188(4183):68–70. doi: 10.1126/science.1114343. [DOI] [PubMed] [Google Scholar]
- Braunstein P. W., Cuénoud H. F., Joris I., Majno G. Platelets, fibroblasts, and inflammation: tissue reactions to platelets injected subcutaneously. Am J Pathol. 1980 Apr;99(1):53–66. [PMC free article] [PubMed] [Google Scholar]
- Feig L. A., Bellvé A. R., Erickson N. H., Klagsbrun M. Sertoli cells contain a mitogenic polypeptide. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4774–4778. doi: 10.1073/pnas.77.8.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn K. C., Carney D. H., Fenton J. W., 2nd, Cunningham D. D. Thrombin active site regions required for fibroblast receptor binding and initiation of cell division. J Biol Chem. 1980 Jul 25;255(14):6609–6616. [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Growth of normal human glial cells in a defined medium containing platelet-derived growth factor. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6611–6615. doi: 10.1073/pnas.77.11.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings T., Jones R. D., Lipton A. A growth factor from spinal cord. J Cell Physiol. 1979 Aug;100(2):273–278. doi: 10.1002/jcp.1041000208. [DOI] [PubMed] [Google Scholar]
- Kaplan K. L., Broekman M. J., Chernoff A., Lesznik G. R., Drillings M. Platelet alpha-granule proteins: studies on release and subcellular localization. Blood. 1979 Apr;53(4):604–618. [PubMed] [Google Scholar]
- Klagsbrun M., Neumann J. The serum-free growth of Balb/c 3T3 cells in medium supplemented with bovine colostrum. J Supramol Struct. 1979;11(3):349–359. doi: 10.1002/jss.400110310. [DOI] [PubMed] [Google Scholar]
- Levine S. P., Krentz L. S. Development of a radioimmunoassay for human platelet factor 4. Thromb Res. 1977 Nov;11(5):673–686. doi: 10.1016/0049-3848(77)90025-1. [DOI] [PubMed] [Google Scholar]
- Paul D., Niewiarowski S., Varma K. G., Rucinski B., Rucker S., Lange E. Human platelet basic protein associated with antiheparin and mitogenic activities: purification and partial characterization. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5914–5918. doi: 10.1073/pnas.77.10.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides P. E., Böhlen P. The mitogenic activity of insulin: an intrinsic property of the molecule. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1138–1144. doi: 10.1016/0006-291x(80)91591-0. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Todaro G. J., Fonte V. A scanning electron microscope study of surface features of viral and spontaneous transformants of mouse Balb-3T3 cells. J Cell Biol. 1973 Dec;59(3):633–642. doi: 10.1083/jcb.59.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Nist C., Kariya B., Rivest M. J., Raines E., Callis J. Physiological quiescence in plasma-derived serum: influence of platelet-derived growth factor on cell growth in culture. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):497–508. doi: 10.1002/jcp.1040970325. [DOI] [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Mendoza S. Monovalent ion fluxes and the control of cell proliferation in cultured fibroblasts. Ann N Y Acad Sci. 1980;339:175–190. doi: 10.1111/j.1749-6632.1980.tb15977.x. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Pledger W. J., Martin P., Antoniades H., Stiles C. D. Transforming viruses directly reduce the cellular growth requirement for a platelet derived growth factor. J Cell Physiol. 1978 Dec;97(3 Pt 1):371–380. doi: 10.1002/jcp.1040970312. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Thomas K. A., Riley M. C., Lemmon S. K., Baglan N. C., Bradshaw R. A. Brain fibroblast growth factor: nonidentity with myelin basic protein fragments. J Biol Chem. 1980 Jun 25;255(12):5517–5520. [PubMed] [Google Scholar]
- Vogel A., Ross R., Raines E. Role of serum components in density-dependent inhibition of growth of cells in culture. Platelet-derived growth factor is the major serum determinant of saturation density. J Cell Biol. 1980 May;85(2):377–385. doi: 10.1083/jcb.85.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]


