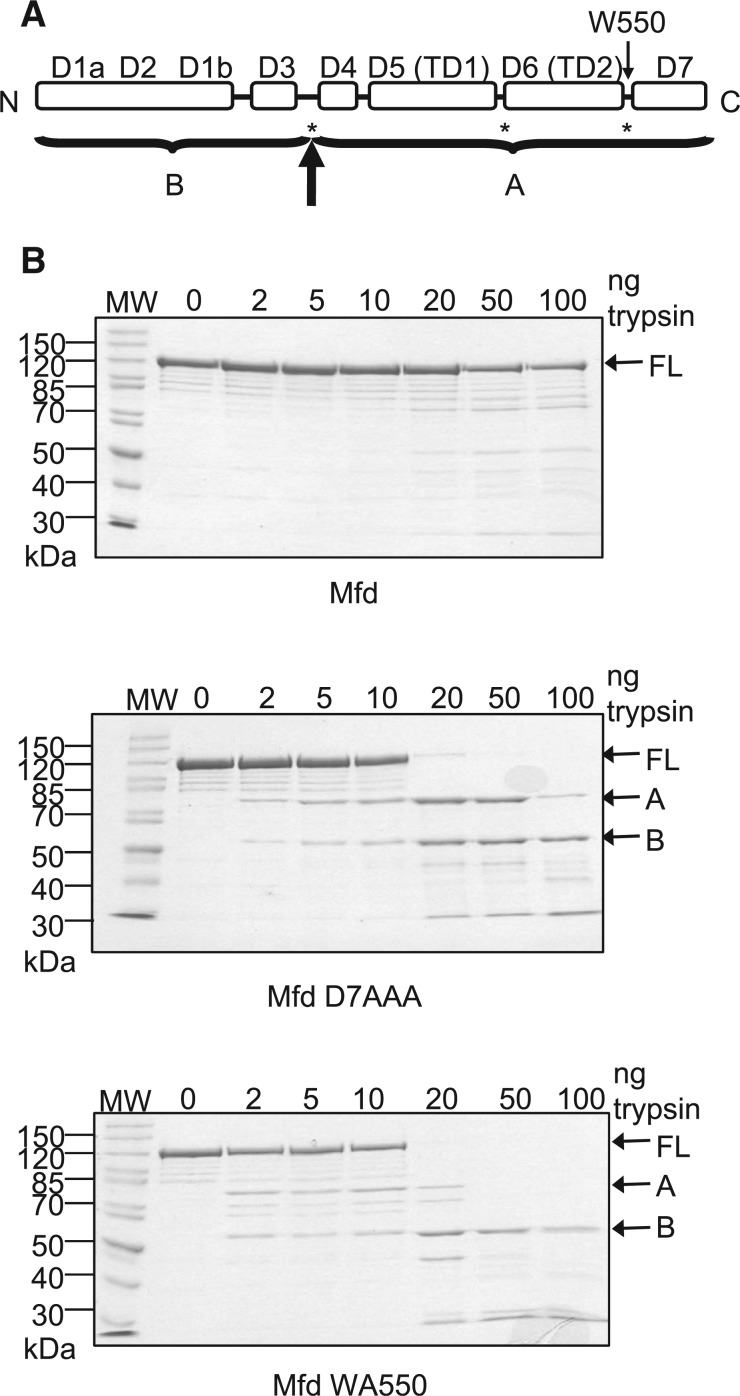
Figure 5.
Effects of disruption of the hook:relay helix interface on the proteolysis of Mfd. (A) Schematic of the domain architecture of Mfd. Asterisks indicate the major sites of trypsin cleavage previously observed in derepressed Mfd mutants in which the D2–D7 interface is disrupted (15). The bold arrow indicates the major site of trypsin cleavage observed in these experiments, with A and B indicating the major proteolysis products that were obtained. (B) Limited trypsin proteolysis of WT Mfd, Mfd D7AAA and Mfd WA550. 2 µM protein was incubated with the indicated amounts of trypsin for 20 min at room temperature and resolved on a 10% SDS-PAGE gel. Gels were stained with Coomassie blue and the images shown are of typical gels. The molecular weights of the molecular weight markers (MW) are indicated in kDa. The position of the full-length protein (FL) and the two major proteolysis products (A and B) are indicated.