Table 5.
Scope of the allylic electrophile
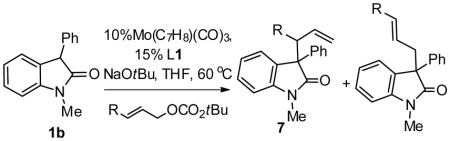
| |||||
|---|---|---|---|---|---|
| entry | R | yield | b/l | dr | ee% |
| 1 | 3,4-Cl2-C6H3 | 87% | 6:1 | 5.5:1 | 89 |
| 2 | 4-OTBS-C6H4 | 90% | 19:1 | 8:1 | 89 |
| 3 | 2-furyl | 92% | 12:1 | 6:1 | 91 |
| 4 | 2-thiophenyl | 88% | 13:1 | 8:1 | 90 |
| 5 | 2NHBocC6H4 | 89% | 19:1 | 19:1 | 93 |
| 6 | 2-(E)-butene | 84% | 6:1 | 6:1 | 91 |
The reaction was performed with 0.1 mmol of oxindole 1b and 0.12 mmol of cinnamyl carbonate. The branch/linear ratio (b/l) and the diastereoselectivity of the branched product(dr) was determined by 1H NMR integration of the crude reaction mixture. The ee% was determined by chiral HPLC.
