Abstract
Background
Protocols for the hormonal induction of ovulation and oviposition are essential tools for managing threatened amphibians with assisted reproduction, but responses vary greatly between species and even broad taxon groups. Consequently, it is necessary to assess effectiveness of such protocols in representative species when new taxa become targets for induction. The threatened genus Mixophyes (family Myobatrachidae) has amongst the highest proportion of endangered species of all the Australian amphibians. This study developed and optimised the induction of oviposition in a non-threatened member of this taxon, the great barred frog (Mixophyes fasciolatus).
Methods
Gravid female M. fasciolatus were induced to oviposit on one or more occasions by administration of human chorionic gonadotropin (hCG) with or without priming with pregnant mare serum gonadotropin (PMSG). Treatments involved variations in hormone doses and combinations (administered via injection into the dorsal lymph sacs), and timing of administration. Pituitary homogenates from an unrelated bufonid species (Rhinella marina) were also examined with hCG.
Results
When injected alone, hCG (900 to 1400 IU) induced oviposition. However, priming with two time dependent doses of PMSG (50 IU, 25 IU) increased responses, with lower doses of hCG (200 IU). Priming increased response rates in females from around 30% (hCG alone) to more than 50% (p = 0.035), and up to 67%. Increasing the interval between the first PMSG dose and first hCG dose from 3 to 6 days also produced significant improvement (p<0.001). Heterologous pituitary extracts administered with hCG were no more effective than hCG alone (p = 0.628).
Conclusions
This study found that M. fasciolatus is amongst the few amphibian species (including Xenopus (Silurana) and some bufonids) that respond well to the induction of ovulation utilising mammalian gonadotropins (hCG). The optimal protocol for M. fasciolatus involved two priming doses of PMSG (50 IU and 25 IU) administered at 6 and 4 days respectively, prior to two doses of hCG (100 IU), 24 hours apart. This study is also the first to demonstrate in an amphibian species that responds to mammalian gonadotropins that an increase in the ovulation rate occurs after priming with a gonadotropin (PMSG) with FSH activity.
Keywords: Amphibian, Mixophyes, Ovulation, Oviposition, Assisted reproduction, hCG, PMSG
Background
Pituitary extracts have been widely used for many decades as an effective and practical tool for obtaining viable oocytes from gravid amphibians [1-6] since the first bioassays demonstrated the effects of pituitary extracts on amphibian ovulation. Pituitary extracts from the same (homologous) species are the most effective at inducing ovulation [2]. Homologous pituitaries are presumably the most suitable and potent source of LH activity [2] for the induction of ovulation because of sequence variation in the active β unit of the gonadotropins [7] that impacts on hormone-receptor specificity, an effect that is likely to increase with phylogenetic distance. Heterologous pituitary extracts from different amphibian species are a “second best” source of LH activity [5] but still (with exceptions such as Xenopus, Table 1) much more effective than phylogenetically distant mammalian gonadotropins [2,8]. However, disadvantages of using heterologous or homologous pituitary extracts as a routine tool for inducing ovulation and oviposition in gravid females include individual, gender and seasonal variability in FSH and LH activity [6], availability of a source of pituitaries, and the cost and inconvenience of the collection process. In the case of endangered species, the potential for transfer of pathogens in raw extracts [9] as well as the inappropriateness of using rare or endangered animals to obtain pituitary extracts argues for alternative induction protocols.
Table 1.
Published protocols for the induction of oviposition inXenopus
| Species | PMSG priming dose (FSH activity)* | hCG priming dose (LH activity)* | “Ovulatory” hCG (dose or source) on Day 1** | Oviposition*** | References |
|---|---|---|---|---|---|
|
Xenopus laevis |
|
|
Human pregnant urine |
Time not stated |
[2,10] |
|
X. laevis |
|
|
90 IU |
Time not stated |
[11] |
|
X. laevis |
|
|
100-200 IU (< 100 g bwt) |
+ 6–12 hrs |
[12] |
| 200–600 IU (> 100 g bwt) | |||||
|
X. laevis |
|
|
300 IU |
Time not stated |
[13] |
|
X. laevis |
|
|
500 IU |
+ 8–10 hrs |
[14] |
|
X. laevis |
|
|
500 IU |
Time not stated |
[15] |
|
X. laevis |
|
|
500-700 IU |
Day 1 |
[16] |
|
X. laevis |
|
|
750 IU |
+ 8–12 hrs |
[17] |
|
X. laevis |
|
|
900 IU |
+ 8–10 hrs |
[18] |
|
X. laevis |
|
|
1000 IU |
+ 10 hrs |
[19] |
|
X. laevis |
|
|
1000 IU |
+ 18 hrs |
[20] |
|
X. laevis |
|
50 IU, Day −14 to −5 (early ovulation if priming after day −5; priming effective up to 1 month) |
500-800 IU |
+ 9–14 hrs |
[21] |
|
X. laevis |
|
50-200 IU, Day 1 (−8 hrs) |
300-500 IU; Day 1 (0 hrs) |
+ 8 hrs |
[22] |
|
X. laevis |
40 IU, Day −4 to −3 |
|
250-500 IU |
+ 6–8 hrs |
[23] |
|
X. laevis |
50 IU, Day? |
|
700 IU |
Time not stated |
[24] |
|
X. laevis |
50 IU, Day −5 to −3 |
|
500 IU |
+ 12–14 hrs |
[25] |
|
X. laevis |
50 IU, Day −17 25 IU, Day −14 |
|
500 IU (hCG inducible over 14 days from second PMSG) |
+ 16–18 hrs |
[26] |
|
X. laevis |
100 IU, Day −1 |
|
500 IU |
Day 2, avg 68% |
[27] |
|
X. tropicalis |
|
20 IU, Day −2 |
100 IU |
Natural pairing |
[28] |
| X. tropicalis | 15 IU, Day −4 to −3 | 150 IU | + 4 hrs | [29] |
Protocols for the induction of oviposition of Xenopus (X. laevis and X. (Silurana) tropicalis) utilising hCG with, or without, priming with mammalian gonadotropins with FSH or LH activity. * Day of administration of priming dose of PMSG or hCG expressed as days before the main “ovulatory” hCG dose (expressed as negative values). ** Day of administration of “ovulatory” hCG dose expressed as Day 1 to facilitate comparison between studies listed in Tables 1 and 2; hCG administered as either reconstituted or synthetic hCG (dose in IU) or as direct injection of pregnant human urine (dose unknown) *** Time or day of oviposition (generally, hours after the main “ovulatory” dose of hCG on Day 1). Species names are from original papers, however, Xenopus tropicalis is now known as Silurana tropicalis.
One great advantage of working with Xenopus spp (X. laevis and X. (Silurana) tropicalis) in reproductive and developmental biology studies involving amphibians has been the ease with which ovulation of fertile eggs can be induced with mammalian gonadotropic hormones, especially hCG as a source of LH activity [8] (Table 1). Xenopus gained widespread attention in the 1930s [10] providing the first effective, large scale pregnancy test for women because of the reliability of its ovulatory response to hCG administration (an application that persisted for many years). This turned out to be an aspect of Xenopus biology shared with few other amphibians (Table 2). The poor response to mammalian gonadotropins in most species (Table 2) and the desire to move away from pituitary extracts for reasons outlined above has led to the development of protocols based on other parts of the hypothalamic-pituitary-gonadal axis. These include gonadotropic releasing hormones (GnRH) to stimulate release of endogenous gonadotropins [30-32], progesterone to directly induce oocyte maturation and germinal vesicle breakdown [33,34], and dopamine antagonists [35,36], as well as various combinations of these with or without gonadotropins (for recent reviews and discussions see [9,36,37]). Nevertheless, as a first step to inducing ovulation in a novel target species, it is reasonable to test the use of synthetic mammalian gonadotropins, to determine whether these are an effective and inexpensive means of obtaining viable eggs, as is the case in Xenopus.
Table 2.
Results of attempts to induce oviposition in amphibians other thanXenopuswith hCG
| Species | Priming or co-administered agent | hCG [dose or source]* | Oviposition** | References |
|---|---|---|---|---|
|
Bufo americanus |
|
Human pregnant urine |
None |
[2] |
|
B. americanus |
No priming |
100 IU, Day 1 |
None |
[38] |
| No priming |
400-1000 IU, Day 1 |
about 60%, Day 2 |
||
| Priming Day −2 [LH 50 ug, or LHRH 50 ug, or eCG 50 IU, or hCG 50 IU] |
500 IU, Day 1 |
≤ 60%, Day 2 |
||
|
B. arenarum |
|
Human pregnant urine |
None |
[2] |
|
B. baxteri |
4.0 ug LHRHa, Day 1 [no priming] |
500 IU, Day 1 |
None |
[35] |
| 0.8 ug LHRHa, Day −4 |
100 IU, Day −4 |
70%, Day 1-2 |
||
| 4.0 ug LHRHa, Day 1 |
500 IU, Day 1 |
|||
| [total = 600 IU] | ||||
| 4.0 ug LHRHa, Day −6 |
500 IU, Day −6 |
80%, Day 1-2 |
||
| 0.8 ug LHRHa, Day −4 |
100 IU, Day −4 |
|||
| 4.0 ug LHRHa, Day 1 |
500 IU, Day 1 |
|||
| [total = 1100 IU] | ||||
|
B. calamita |
|
Human pregnant urine |
None |
[2] |
|
B. fowleri |
|
Human pregnant urine |
+ |
[2] |
|
B. fowleri |
60 ug LHRHa, 5 mg progesterone, 0.25 mg pimozide, Day 1 |
500 IU, Day 1 |
85%, Day 1-2 |
[39] |
| 4 ug LHRHa, Day −1 |
500 IU, Day −1 |
29%, Day 1-2 |
||
| 4 ug LHRHa, Day 1 |
500 IU, Day 1 |
|||
| [total = 1000 IU] | ||||
|
B. vulgaris |
|
Human pregnant urine |
None |
[2] |
|
Eleutherodactylus coqui |
|
25-140 IU, Day 1 |
None |
[40] |
| 165-200 IU, Day 1 |
Day 2, weak response (2/6 females) |
|||
|
Hyla aurea |
Pregnant mare serum |
- |
+ |
[2] |
|
Litoria aurea |
5 ug LHRHa, Day −4.5 |
300 IU, Day 1 |
Weak; 1% of normal egg release (2/5 females) |
[41] |
| 5 ug LHRHa, Day −1.5 | ||||
| 10 ug LHRHa, Day −0.5 | ||||
| 20 ug LHRHa, Day 1 | ||||
|
Litoria moorei |
No benefit of including progesterone |
100-200 IU, Day −2 |
Weak; few eggs in 1/14 females |
[41] |
| 500–750 IU, Day 1 | ||||
|
Litoria raniformis |
10 ug LHRHa, Day −4 |
500 IU, Day −4 |
None |
[42] |
| 10 ug LHRHa, Day −2 |
500 IU, Day −2 |
|||
| 10 ug LHRHa, Day 1 |
500 IU, Day 1 |
|||
|
Rana catesbeiana |
|
Human pregnant urine |
+ |
[2] |
|
R. catesbeiana |
Pregnant mare serum |
- |
+ |
[2] |
|
R. clamitans |
|
Human pregnant urine |
None |
[2] |
|
R. temporaria |
|
Human pregnant urine |
None |
[2] |
|
R. esculenta |
|
Human pregnant urine |
None |
[2] |
|
R. pipiens |
|
Human pregnant urine |
None |
[2] |
|
R. pipiens |
Pregnant mare serum |
- |
None |
[2] |
|
R. sevosa |
3-4 ug LHRH, Day −3 |
100 IU, Day −3 |
+ |
[37] |
| 15–20 ug LHRH, Day 1 |
500 IU, Day 1 |
|||
|
R. vulgaris |
|
Human pregnant urine |
None |
[2] |
|
Urodeles |
|
|
|
|
|
Ambystoma tigrinum |
|
Human pregnant urine |
+ |
[2] |
|
Triturus pyrrogaster |
|
Human pregnant urine |
+ |
[2] |
|
T. torosus; |
Pregnant mare serum |
- |
None |
[2] |
|
T. similans; | ||||
|
T. rivularis | ||||
| T. viridescens | Human pregnant urine | + | [2] |
Results of attempts to induce oviposition utilising hCG administration in amphibian species other than Xenopus with, or without, priming with hCG or other agents. * Day of final hCG injection expressed as Day 1, with administration of compounds on days prior to Day 1 expressed as negative values (to facilitate comparison between studies listed in Tables 1 and 2); hCG administered as either reconstituted or synthetic hCG (dose in IU) or as direct injection of pregnant human urine (dose unknown). ** Oviposition: “+” = oviposition recorded; “None” = no oviposition recorded. Data from [2] for PMSG only inductions (no hCG) for some Rana species also shown. Species names are from original papers, however, some have since been renamed e.g. Rana pipiens now Lithobates pipiens by some [43].
Global declines since the 1960s have resulted in amphibians experiencing the highest rate of decline and extinction of any vertebrate class over that period [44-46]. This loss of amphibian biodiversity is primarily a function of a global pandemic of chytridiomycosis. Amongst Australian frogs, the genus Mixophyes (commonly known as the Barred Frogs) is amongst the most threatened genera. Currently, >40% (3 out of 7) of extant Mixophyes species are listed as vulnerable or endangered under Australian federal and state legislation, as well as the IUCN Red List. In the state of New South Wales, this figure is as high as 75% (3 out of 4) species of Mixophyes listed as endangered or vulnerable. Consequently, protocols for assisted reproduction, such as hormonal induction of ovulation have the potential to contribute to management and conservation of species across the genus. Currently, there is no data available for induced ovulation in gravid females of Mixophyes species. Adding to this, the group (Family: Myobatrachidae) is phylogenetically distant from many other amphibian taxa, and may consequently be quite different in its response to protocols that work well for other amphibian taxa, including its response to the injection of pituitary homogenates from unrelated species. Collectively the myobatrachids constitute about 50% of Australian amphibian species; 22% (n = 23) are considered vulnerable or endangered with 3 (2.8%) considered extinct; and they also constitute 48% of all threatened Australian frogs [47].
This paper reports the outcome of analyses of data on multiple ovulation and oviposition induction protocols employed on Mixophyes fasciolatus (the great barred frog). This species is the only non-threatened species of the Mixophyes in New South Wales and one of the few non-threatened species in Australia, and as such is an appropriate model for establishing protocols for assisted reproductive techniques (ART) for this genus. The data set does not contain all feasible protocols for the induction of ovulation (e.g. no data is available for effects of GnRHs or dopamine antagonists), but has focussed on the use of amphibian pituitary homogenates and mammalian FSH and LH preparations.
The results have implications for optimising oocyte collection in the other threatened Mixophyes species and, more broadly, other Australian ground (myobatrachid and limnodynastid) frogs. The results also demonstrate that, in species that respond to hCG, PMSG priming is effective in increasing the rate of ovulation (an assumption in some published protocols, not previously substantiated with data).
Methods
Research described in this manuscript was undertaken following approval by University of Newcastle Animal Care and Ethics Committee which adheres to the NSW Animal Research Act, NSW Animal Research Regulation, and the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (706 06 08 to 706 06 10), and M. fasciolatus were collected under permit from the NSW National Parks and Wildlife Service.
Sources and holding of animals
Adult female Mixophyes fasciolatus (great barred frogs) were collected from the mid to north coast regions of New South Wales, Australia (32o59’36.95”S; 151o26’44.23”E to 28o33’08.51”S; 153o18”31.72”E) during the spring to autumn periods (September to April) and held in groups of 10–12 in large plastic containers (approximately 1 m × 2 m × 1 m), with refuge sites provided as deep pine bark, leaf litter, wood and eucalypt bark. Access to water and food (brown crickets, Acheta domestica) was provided ad libitum, and environmental conditions (temperature and day length) were partially regulated by air-conditioning and fluorescent lighting in a facility that received partial lighting through external glass windows. Ambient temperatures varied between 16 and 28°C; light: dark cycle was approximately 10 L: 14 D.
Individual females were identified by implanted Passive Integrated Transponder (PIT) tags (GuangZhou HongTeng Barcode Technology Co. Ltd; Guangzhou). Females were assigned randomly to treatments (oviposition induction protocols), and females were used between the months of January and April over a period of two years. Induction protocols are described below. A proportion of females were subjected to induction attempts on more than one occasion, but were rested for at least 2 months between inductions (but generally longer than this, and up to 1 year). No prior information was available on either the seasonality of oogenesis, or the rate at which successive generations of mature oocytes are recruited to follicles in this species.
Induction protocols
Gravid, adult females were subjected to a number of induction protocols, as indicated in Table 3. These were:
Table 3.
Oviposition by femaleM. fasciolatusafter various mammalian gonadotropin treatments
| Treatment groups | Day, time & number of females ovipositing | Oviposition (%)* | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Day |
1 |
2 |
3 |
4 |
5 |
6 |
|
|||||||||||||||
|
Light/dark (24 hr)** |
D |
L |
L |
D |
D |
L |
L |
D |
D |
L |
L |
D |
D |
L |
L |
D |
D |
L |
L |
D |
D |
|
|
(1) Control – Saline only: 4 injections | ||||||||||||||||||||||
|
Saline |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0/6 (0%) |
|
(2) hCG only | ||||||||||||||||||||||
|
hCG 900 |
|
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
|
|
|
|
|
6/20 (30%) |
|
hCG 1050-1400 |
|
|
|
|
|
|
|
|
1 |
|
|
|
1 |
1 |
1 |
|
|
|
|
|
|
4/17 (23.5%) |
|
All hCG only |
|
10/37 (27%) |
||||||||||||||||||||
|
(3) hCG and pituitary glands | ||||||||||||||||||||||
|
hCG 1200–1500 + 6 pituitaries |
|
|
|
|
|
|
|
|
1 |
1 |
|
|
|
|
|
|
|
|
|
|
|
2/7 (29%) |
|
(4) PMSG and hCG (200 IU***) | ||||||||||||||||||||||
|
50 PMSG; -3 days |
|
|
|
|
|
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1/6 (17%) |
|
75 PMSG;-4, -2 days**** |
|
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
|
4/12 (33%) |
|
75 PMSG;-5, -3 days**** |
|
|
|
|
|
|
|
|
6 |
6 |
|
1 |
6 |
|
|
|
1 |
|
|
|
1 |
21/41 (51%) |
|
75 PMSG;-6, -4 days**** |
|
|
|
|
1 |
|
|
1 |
1 |
|
|
|
1 |
1 |
|
|
1 |
|
|
|
|
6/9 (67%) |
| All PMSG and hCG (200 IU) | 32/68 (47%) | |||||||||||||||||||||
Oviposition by female M. fasciolatus after either (1) sham injections with saline (2) injections with human chorionic gonadotropin (hCG) only (3) injections with human gonadotropin and 6 amphibian pituitary glands (B. marinus) (4) injection with pregnant mare serum gonadotropin (PMSG) indicated number of days before administration of first of two doses of human chorionic gonadotropin. * Number ovipositing/Number treated (% ovipositing) ** Light/Dark cycle = 12 hr L: 12 hr D; each L or D represents 6 hrs ***hCG = 100 IU administered on each of days 1 and 2. **** Where PMSG total dose = 75 IU: first dose = 50 IU; second dose = 25 IU administered on the indicated number of days before the first dose of hCG.
1. Controls given sham 250 μl saline injections (Simplified Amphibian Ringer (SAR); [6]) once per day for four days.
2. Varying cumulative doses of human chorionic gonadotropin (hCG) (Chorulon, Intervet (300 IU/ml); these were administered in the manufacturer’s diluent over up to three days (mostly over two days) in up to 4 aliquots to deliver the total doses indicated in Table 3.
3. hCG in combination with pituitary gland extracts from adult male cane toads (Rhinella marina); each female received pituitary extract injections on two consecutive days, each containing extracts from 3 pituitary glands in 250 μl of SAR, and total hCG injections (300 IU/ml in manufacturer’s diluent) over up to 3 days as indicated in Table 3.
4. hCG preceded with priming injections of pregnant mare serum gonadotropin Pregnant mare serum gonadotropin (PMSG) (Folligon, Intervet, 200 IU/ml in the manufacturer’s diluent) was administered between 2 and 6 days prior to the first hCG injection; 200 IU hCG was administered as two 100 IU aliquots administered separately on days 1 and 2 (Table 3), with the exception of a small number of females where the hCG injections were separated by 48 hours.
During the experimental period, females were maintained in individual vivaria on a 12 D: 12 L photoperiod at room temperature. Compounds were administered by injection into the dorsal lymph sacs via either a 23 or 26 G hypodermic needle.
Multiple inductions
About 50% of the females were used in induction protocols on more than one occasion. Females used in more than one induction were rested for at least 2 months between induction attempts (but generally for longer), whether or not oviposition was observed in any preceding induction attempt. Females used in more than one induction attempt were randomly assigned to induction protocols on the second or third induction i.e. there was no systematic sequence, combination or set of induction protocols used where females were induced more than once. Thus, the data in Table 4 represents responses to multiple induction attempts employing various combinations of protocols (without systematic assignment).
Table 4.
Effect of diurnal cycle on timing of oviposition byM. fasciolatus
| |
Treatment Group/Number of ovipositions observed |
|||
|---|---|---|---|---|
| Oviposition time (diurnal cycle) | hCG only | hCG and pituitary glands | PMSG and hCG | N |
|
0 – 12 hours* |
9 |
2 |
30 |
41 |
| 12 – 24 hours** | 1 | 0 | 2 | 3 |
Effect of diurnal cycle on timing of oviposition by M. fasciolatus. Data from Table 3. No significant effect of treatment group on timing of oviposition (p = 0.625); significant effect of stage of diurnal cycle on oviposition timing (p<0.0001). * 0–12 hours = 0:00–6:00 hr dark, 6:00–12:00 hr light; **12-24 hours = 12:00–18:00 hr light, 18:00–24:00 hr dark.
Measures of response
Voluntary oviposition by females was used as the measure of response to induction protocols. Responses were recorded as occurring or not occurring. After injections, females were held in individual plastic vivaria for several days to assess their responses. Oviposition was recorded as oocytes deposited on the floor of vivaria; a small amount of isotonic saline being added to the vivaria around the time of oviposition to prevent desiccation of oocytes. Prior to oviposition, females were hydrated by small amounts of water added to the vivaria. Oocytes were not collected by manual stimulation. Most ovipositions involved the release of large numbers of oocytes (ranging from several hundred up to approximately 1000). A small number of ovipositions were associated with the release of fewer oocytes. The viability of several batches of oocytes was confirmed by in vitro fertilisation with sperm of male M. fasciolatus, with observations to the neurula stage. It is assumed in this study that oviposition indicates the occurrence of ovulation immediately prior to oviposition (as a result of the hormonal induction protocols), although no experiments were done to directly demonstrate this.
Statistics
Data on the frequency of oviposition between treatments and treatment groups were analysed using non-parametric statistics. To avoid problems with expected frequencies less than 5, all frequency statistics were analysed using Fisher Exact Probability Tests ( http://vassarstats.net/; last accessed July 8, 2012), and employing the Freeman-Halton extension for contingency tables greater than 2 × 2. Data for the proportion of PMSG primed females ovipositing were plotted against time since first PMSG injection and the significance of the regression and correlation coefficients determined by least squares regression analysis.
Results
A comparison of protocols
Controls (Group 1, Table3) No ovipositions were recorded in females subjected to saline only injections. This supports the conclusion that where ovipositions were recorded in hormonal treatment groups, these were a result of those treatments (p = 0.056, Table 5).
Table 5.
Matrix of Fisher exact test p values (one tailed) from data in Table3
| All treatments | hCG only | hCG and pituitary glands | PMSG and hCG | |
|---|---|---|---|---|
|
Saline Control |
0.056 (6,112) |
0.182 (6,37) |
0.269 (6, 7) |
0.028 (6,68) |
|
hCG only |
|
- |
0.628 (37,7) |
0.035 (37,68) |
| hCG and pituitary glands | - | - | 0.300 (7,68) |
Matrix of Fisher exact test p values (one tailed) from data in Table 3 for comparisons between treatment groups of proportion of female M. fasciolatus ovipositing. Sample sizes of tested groups shown in parentheses.
hCG alone (Group 2, Table3) Administration of hCG alone was shown to induce oviposition with doses totalling 900 to 1400 IU per female (Table 3). The overall oviposition rate in hCG only females was 27%. There was no evidence (p = 0.512) that increasing the total dose above 900 IU increased the rate of oviposition when hCG was the only treatment. hCG doses below 900 IU without the administration of other compounds were not tested in this study. Ovipositions with hCG alone were recorded up to 4 days after the initial injections, although most occurred by the end of the night of the second day.
hCG and pituitary glands (Group 3, Table3) The overall oviposition rate in females treated with hCG and pituitary gland extracts was 29% (Table 3), which was not significantly different from the proportion of females ovipositing following hCG only treatments (p = 0.628, Table 5). No females were administered pituitary gland extracts without hCG in this study. All ovipositions occurred by the end of the night of the second day after the first injections.
hCG following PMSG priming (Group 4, Table3) Some ovipositions with PMSG priming occurred earlier than any ovipositions with hCG alone. Taken together, the rate of oviposition in females subjected to PMSG priming prior to hCG injections (47%, Table 3) was significantly higher than in females subjected to hCG only (27%, Table 3; p = 0.035, Table 5). Ovipositions were recorded up to 6 days after the initial hCG injection, but most occurred from the morning of the third day through to the morning of the fourth day after the first hCG injection.
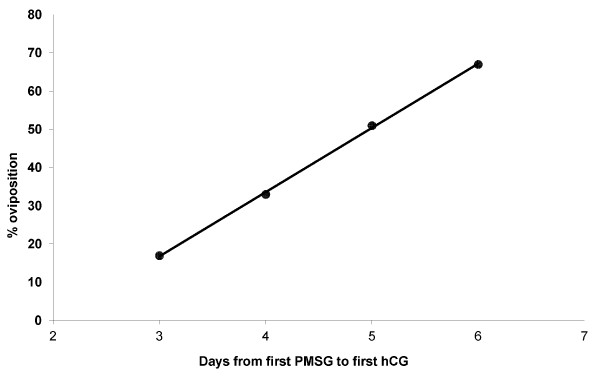
There was a significant increase (p<0.001) in the rate of oviposition as a function of the time between the first PMSG injection and the first hCG injection, based on the regression of the percentage oviposition rate against the interval between first PMSG and first hCG (Figure 1) i.e. the oviposition rate was higher when PMSG priming began 5 to 6 days before first hCG than when it began closer to the first day of hCG administration.
Figure 1.
Regression of mean% femaleM. fasciolatusovipositing against time from first PMG injection to first hCG injection. Data from Treatment Group “PMSG and hCG” in Table 3 (R2 = 0.9994; p<0.001).
Repeated (multiple) inductions
Data from Table 6 shows the response of females to induction attempts performed on more than one occasion. 5/20 (25%) females in which inductions were attempted twice, ovulated twice. This indicates the capacity of females to go through multiple ovarian cycles and repeated ovulation and oviposition events in captivity. The total proportion of females subjected to induction protocols that oviposited at least once, increased with multiple induction attempts to 100% by the third attempt (Table 6); this trend of increasing proportion of females ovipositing at least once with repeated induction attempts indicates most, if not all, females held were in reproductive condition at some point, if not continuously over the duration of the study.
Table 6.
Proportions of femaleM. fasciolatusovipositing after repeated induction attempts
| Number of times induction attempted | 1 | 2 | 3 |
|---|---|---|---|
|
Number of females |
29 |
20 |
6 |
|
Number of females ovipositing at least once |
16 |
13 |
6 |
|
% females ovipositing at least once |
55%a |
65%a,b |
100%b |
| Number of females ovipositing twice | 5 (25%) | 0 |
Capacity of female M. fasciolatus held under captive conditions to oviposit after induction attempts on more than one occasion. The data are pooled from all induction attempts across all protocols. Repeated attempts at induction of oviposition were separated by resting periods of at least 2 months. Percentages with different superscripts on the same row are significantly (p<0.05) different.
Timing of oviposition
Oviposition occurred most frequently in the period including the second half of the dark period (0:00–6:00 hrs, Table 3) and the first half of the light period (6:00–12:00 hours, Table 3) of the daily photoperiod cycle, suggesting a behavioural orientation towards nocturnal oviposition (p<0.0001; Table 4) that may continue early into the light period.
Discussion
This study adds M. fasciolatus to the small group of amphibian species (including Xenopus laevis, Silurana tropicalis, and some bufonids) that will ovulate and oviposit in response to protocols based only on mammalian gonadotropins. The range of hCG doses associated with successful oviposition in M. fasciolatus are similar to the range of doses reported in various Xenopus/Silurana protocols (this study up to 1400 IU, but optimised to 200 IU with PMSG priming; compared to Xenopus/Silurana 90 to 1000 IU without FSH/PMSG priming, 250 to 500 IU with priming; Table 1). The absolute dose for M. fasciolatus (not adjusted for body weight differences) is similar or less than that required to induce B. americanus and B. fowleri (Table 2). It is assumed that oviposition in this study also indicates ovulation occurring as a result of the induction process (as would be the case with most studies investigating the induction of oviposition in amphibians), although this was not tested directly; there are no published data on anurans known to the authors where ovulation and oviposition have been shown not to be linked, sequential events.
In M. fasciolatus there is a distinct benefit of priming with a gonadotropin with FSH activity. It was demonstrated in this study that the rate of ovulation can be increased from around 30% to more than 50% by priming females with PMSG. Interestingly, the benefits of priming were increased by extending the interval between the first PMSG priming dose and the first hCG dose. Based on the data from this study, the optimal protocol for inducing oviposition in M. fasciolatus involves injection of two priming doses of PMSG (50 IU and 25 IU) administered 6 and 4 days, respectively, prior to the injection of two ovulatory doses of 100 IU hCG, administered 24 hours apart.
Published induction protocols utilising hCG (Tables 12) identify Xenopus and Silurana species from the family Pipidae as those that respond most strongly to hCG, administered alone or in combination with a mammalian gonadotropin with FSH priming activity, or with other components of the hypothalamo-pituitary-gonadal pathway (Table 1). The data in Table 1 indicates a wide range of hCG concentrations (from 90 IU to 1000 IU in X. laevis as single hCG doses, a range of an order of magnitude) have been used to induce Xenopus laevis and Silurana tropicalis. An apparent higher sensitivity to hCG by S. tropicalis may reflect its smaller body size [48]. Various protocols in Table 1 have also reported benefits of priming the ovaries with either lower, anovulatory doses of hCG, or with PMSG to prime for maturation. Nevertheless, there do not appear to be reports of data on the generation of dose response curves for induction (hence the wide variation in protocols), even in Xenopus, which might indicate optimal doses that achieve maximum egg generation with the minimum hCG dose, nor which indicate the quantitative impacts of priming with hCG or PMSG. Most published Xenopus/Silurana protocols do not incorporate FSH priming (although a number of protocols have an hCG “priming” dose - see Table 1).
Although some Xenopus/Silurana protocols report priming doses of PMSG (Table 1), the authors of this study are unaware of a direct comparison in any study of protocols with and without priming that would prove a benefit of priming with FSH. This study thus provides the only published data that demonstrates in any amphibian species an increase in ovulation rate as a result of such priming, and that the maturational effect of priming increases with time since administration. FSH is common in mammalian protocols, recognising the role that FSH plays in recruiting follicles and priming the maturing follicle for ovulation by cumulus expansion during the LH induced resumption of meiosis and germinal vesicle breakdown [49,50]. LH but not FSH [51] causes a rise in progesterone in Xenopus follicles, indicating a separate, non-progesterone effect of FSH on follicle maturation.
Many anuran taxa, including various species of ranids, hylids and some bufonids (listed in Table 1) do not respond well (do not ovulate or ovulate at a very low rate) to induction with mammalian homologues of the amphibian gonadotropic hormones (a fact recognised many years ago: see [2,6]). The data in Table 2 shows that, in particular, ranids as a group are the least responsive to mammalian homologues of any higher amphibian taxon that has been studied in detail (resulting in the continued use of pituitary extracts for induction in this group, see below); bufonids are highly variable in their responses – many species have not been recorded as responding to mammalian homologues, others respond in conjunction with other effectors of the hypothalamic-pituitary-gonadal axis such as LHRH and progesterone, while at least two species (B. americanus and B. fowleri) respond reasonably well to hCG only inductions. Some less studied groups such as the Australasian hylids and the eleutherodactylids may or may not be poor responders, with results obtained on a small sample of species. In contrast, a number of urodeles appear to respond well to hCG.
Nevertheless, effective hCG doses for Xenopus and Mixophyes fasciolatus (allowing for differences in body weight) are high compared to doses reported to be effective in inducing ovulation in mammals (mouse 5 IU [52,53], dog 500 IU [54], rhesus monkey 4000 IU [55], cheetah 100–250 IU [56], cows 1500 to 2500 IU [57,58], mares 2,000 – 3,300 IU [59,60]; for example, the optimised dose of hCG used to induce oviposition in M. fasciolatus in this study (200 IU with PMSG priming) would have been sufficient to induce ovulation in 2 cheetahs [56]. This large differential in effective dose between mammals and responsive amphibians is also noted by Kouba and Vance [37] who observed that the ovulatory dose of hCG for Xenopus is 2000 times for that for the tiger. hCG is a convenient source of mammalian LH activity, but other mammalian sources are not necessarily more potent [8].
Given the challenges of inducing ovulation with mammalian homologues, pituitary extracts have continued to be used in many species to induce ovulation including bufonids [61], ranids [3,62,63] and at least one other myobatrachid [4]. No data were generated in this study on the capacity of amphibian pituitary gland extracts alone to induce ovulation and oviposition in M. fasciolatus, and there was no evidence (although the approach was not exhaustively investigated) of pituitary extracts potentiating the effects of the mammalian gonadotropins. The source of amphibian pituitary extracts in this study was Rhinella marina, which is not closely related to the Mixophyes[64]. The effect of homologous pituitary extracts remains untested in this species.
The results of this study do not preclude further work on this or other myobatrachid species to improve the rate and control of ovulation in assisted reproduction approaches. The use of gonadotropin releasing hormone analogues to induce ovulation has been reported in other myobatrachid species, Pseudophryne guentheri[65] and P. corroboree[66]. Investigations focussing on combining gonadotropin releasing hormones and dopamine antagonists [39] might also improve success rates and the control of timing of ovulation and oviposition in M. fasciolatus and other Mixophyes species, as might various combinations of hCG or other gonadotropins with progesterone [35,37].
Mixophyes frogs are one of the most threatened Australian myobatrachid genera (with only Taudactylus containing a higher proportion of threatened species). There is one instance of captive breeding reported in a Mixophyes species (M. fasciolatus at Melbourne Zoo; [67]). In the future, captive breeding approaches in this and other Mixophyes species may be augmented by assisted reproduction using techniques including induced ovulation for in vitro fertilisation that allow specific individuals to be paired for optimal genetic management, and improve efficiency and reduce costs in resource limited captive programs, and play a role in breeding programs selecting for disease resistance, such as against chytridiomycosis.
Conclusions
This study found that M. fasciolatus is amongst the few amphibian species (including Xenopus (Silurana) and some bufonids) that respond well to the induction of ovulation utilising mammalian gonadotropins (hCG). The optimal protocol for M. fasciolatus involved two priming doses of PMSG (50 IU and 25 IU) administered at 6 and 4 days respectively, prior to two doses of hCG (100 IU), 24 hours apart. This study is also the first to demonstrate in an amphibian species that responds to mammalian gonadotropins that an increase in the ovulation rate occurs after priming with a gonadotropin (PMSG) with FSH activity.
Abbreviations
hCG: Human chorionic gonadotropin; PMSG: Pregnant mare serum gonadotropin; LH: Luteinizing hormone; FSH: Follicle-stimulating hormone; GnRH: Gonadotropic releasing hormones; ART: Assisted reproductive techniques; PIT: Passive integrated transponder; SAR: Simplified amphibian ringer.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JC and SC contributed to the design of the study, participated in carrying out the trials, conducted the analysis of the data and interpretation of the findings, and prepared the manuscript. JG, AF and MM contributed to the design of the study, participated in carrying out the trials, and made comment on the manuscript. MA acquired funding for the study, contributed to the design of the study, made comment on the manuscript and helped with aspects of the laboratory work. All authors read and approved the final manuscript.
Contributor Information
John Clulow, Email: john.clulow@newcastle.edu.au.
Simon Clulow, Email: simon.clulow@newcastle.edu.au.
Jitong Guo, Email: gjitong@gmail.com.
Andrew J French, Email: afrench4@optusnet.com.au.
Michael J Mahony, Email: michael.mahony@newcastle.edu.au.
Michael Archer, Email: m.archer@unsw.edu.au.
Acknowledgements
Funding for this project was provided by anonymous donors in the interests of advancing innovative conservation strategies. Carmen McCartney made valuable comments on this manuscript.
References
- Browne RK, Clulow J, Mahony M, Clark A. Successful Recovery of Motility and Fertility of Cryopreserved Cane Toad (Bufo marinus) Sperm. Cryobiology. 1998;37:339–345. doi: 10.1006/cryo.1998.2129. [DOI] [PubMed] [Google Scholar]
- Creaser CW, Gorbman A. Species Specificity of the Gonadotropic Factors in Vertebrates. Q Rev Biol. 1939;14:311–331. doi: 10.1086/394589. [DOI] [Google Scholar]
- Di Berardino MA. In: Methods in Developmental Biology. Wilt FH, Wessells NK, editor. New York: Thomas Y Crowell; 1967. Frogs; pp. 55–74. [Google Scholar]
- Edwards DL, Mahony MJ, Clulow J. Effect of sperm concentration, medium osmolality, and oocyte storage on artificial fertilisation success in a myobatrachid frog (Limnodynastes tasmaniensis) Reprod Fertil Dev. 2004;16:347–354. doi: 10.1071/RD02079. [DOI] [PubMed] [Google Scholar]
- Lofts B. In: Reproduction. Lofts B, editor. New York: Academic; 1974. pp. 107–218. (Physiology of the Amphibia). [Google Scholar]
- Rugh R. Induced Breeding. Minneapolis, Minnesota: Burgess Publishing Company; 1962. pp. 91–103. (Experimental Embryology Techniques and Procedures). [Google Scholar]
- Komoike Y, Ishii S. Cloning of cDNAs encoding the three pituitary glycoprotein hormone β subunit precursor molecules in the Japanese toad, Bufo japonicus. Gen Comp Endocrinol. 2003;132:333–347. doi: 10.1016/S0016-6480(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Licht P, Papkoff H. Species specificity in the response of an in vitro amphibian (Xenopus laevis) ovulation assay to mammalian luteinizing hormones. Gen Comp Endocrinol. 1976;29:552–555. doi: 10.1016/0016-6480(76)90039-3. [DOI] [PubMed] [Google Scholar]
- Kouba AJ, Vance CK, Willis EL. Artificial fertilization for amphibian conservation: current knowledge and future considerations. Theriogenology. 2009;71:214–227. doi: 10.1016/j.theriogenology.2008.09.055. [DOI] [PubMed] [Google Scholar]
- Gurdon J, Hopwood N. The introduction of Xenopus laevis into developmental biology: of empire, pregnancy testing and ribosomal genes. Int J Dev Biol. 2000;44:43–50. [PubMed] [Google Scholar]
- Barr WA, Hobson BM, Di Vita G. Method for estimating the Number of Eggs laid by Xenopus laevis in Response to the Injection of Gonadotrophin. Nature. 1967;214:827–828. doi: 10.1038/214827a0. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Hedrick JL. A molecular approach to fertilization: II. Viability and artificial fertilization of Xenopus laevis gametes. Dev Biol. 1971;25:348–359. doi: 10.1016/0012-1606(71)90036-4. [DOI] [PubMed] [Google Scholar]
- Elsdale TR, Gurdon JB, Fischberg M. A Description of the Technique for Nuclear Transplantation in Xenopus laevis. J Embryol Exp Morphol. 1960;8:437–444. [PubMed] [Google Scholar]
- Katagiri C, Yoshizaki N, Kotani M, Kubo H. Analyses of Oviductal Pars Recta-Induced Fertilizability of Coelomic Eggs in Xenopus laevis. Dev Biol. 1999;210:269–276. doi: 10.1006/dbio.1999.9285. [DOI] [PubMed] [Google Scholar]
- Freeman S. A study of the jelly envelopes surrounding the egg of the amphibian, Xenopus laevis. Biol Bull. 1968;135:501–513. doi: 10.2307/1539712. [DOI] [Google Scholar]
- Hollinger TG, Corton GL. Artificial fertilization of gametes from the South African clawed frog, Xenopus laevis. Gamete Res. 1980;3:45–57. doi: 10.1002/mrd.1120030106. [DOI] [Google Scholar]
- Olson JH, Chandler DE. Xenopus laevis Egg Jelly Contains Small Proteins That Are Essential to Fertilization. Dev Biol. 1999;210:401–410. doi: 10.1006/dbio.1999.9281. [DOI] [PubMed] [Google Scholar]
- Bonnell BS, Reinhart D, Chandler DE. Xenopus laevis Egg Jelly Coats Consist of Small Diffusible Proteins Bound to a Complex System of Structurally Stable Networks Composed of High-Molecular-Weight Glycoconjugates. Dev Biol. 1996;174:32–42. doi: 10.1006/dbio.1996.0049. [DOI] [PubMed] [Google Scholar]
- Al-Anzi B, Chandler DE. A sperm chemoattractant is released from xenopus egg jelly during spawning. Dev Biol. 1998;198:366–375. [PubMed] [Google Scholar]
- Kampf N, Zohar C, Nussinovitch A. Alginate Coating of Xenopus laevis Embryos. Biotechnol Prog. 2000;16:497–505. doi: 10.1021/bp990153u. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Inducing ovulation in Xenopus laevis. Cold Spring Harbor Protocols. 2007. [DOI] [PubMed]
- Gurdon JB. In: Methods in Developmental Biology. Wilt FH, Wessells NK, editor. New York: Thomas Y Crowell; 1967. African Clawed Frogs; pp. 75–84. [Google Scholar]
- Sato K-i, Tokmakov AA, Iwasaki T, Fukami Y. Tyrosine Kinase-Dependent Activation of Phospholipase Cγ Is Required for Calcium Transient in Xenopus Egg Fertilization. Dev Biol. 2000;224:453–469. doi: 10.1006/dbio.2000.9782. [DOI] [PubMed] [Google Scholar]
- Perez-Mongiovi D, Beckhelling C, Chang P, Ford CC, Houliston E. Nuclei and Microtubule Asters Stimulate Maturation/M Phase Promoting Factor (Mpf) Activation in Xenopus Eggs and Egg Cytoplasmic Extracts. J Cell Biol. 2000;150:963–974. doi: 10.1083/jcb.150.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S, Kroll KL, Amaya E. Generation of Transgenic Xenopus laevis: I. High-Speed Preparation of Egg Extracts. CSH Protocols. 2007. [DOI] [PubMed]
- Desai A, Murray A, Mitchison TJ, Walczak CE. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 1999;61:385–412. doi: 10.1016/s0091-679x(08)61991-3. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Dake J, Iwashina Y-k, Tokumoto T. Induction of ovulation in Xenopus without hCG injection: the effect of adding steroids into the aquatic environment. Reprod Biol Endocrinol. 2011;9:11. doi: 10.1186/1477-7827-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort DJ, Thomas JH, Rogers RL, Noll A, Spaulding CD, Guiney PD, Weeks JA. Evaluation of the Developmental and Reproductive Toxicity of Methoxychlor using an Anuran (Xenopus tropicalis) Chronic Exposure Model. Toxicol Sci. 2004;81:443–453. doi: 10.1093/toxsci/kfh230. [DOI] [PubMed] [Google Scholar]
- Kenwrick S, Amaya E, Papalopulu N. Pilot morpholino screen in Xenopus tropicalis identifies a novel gene involved in head development. Dev Dyn. 2004;229:289–299. doi: 10.1002/dvdy.10440. [DOI] [PubMed] [Google Scholar]
- Daniels E, Licht P. Effects of gonadotropin-releasing hormone on the levels of plasma gonadotrophins (FSH and LH) in the bullfrog, Rana catesbeiana. Gen Comp Endocrinol. 1980;42:455–463. doi: 10.1016/0016-6480(80)90211-7. [DOI] [PubMed] [Google Scholar]
- Hubbard GM, Licht P. In vitro study of the direct ovarian effects of gonadotropin-releasing hormone (GnRH) in the frogs, Rana pipiens and Rana catesbeiana. Gen Comp Endocrinol. 1985;60:154–161. doi: 10.1016/0016-6480(85)90309-0. [DOI] [PubMed] [Google Scholar]
- Porter DA, Licht P. Pituitary responsiveness to superfused GnRH in two species of ranid frogs. Gen Comp Endocrinol. 1985;59:308–315. doi: 10.1016/0016-6480(85)90383-1. [DOI] [PubMed] [Google Scholar]
- Schuetz AW. Induction of oocytic maturation and differentiation: mode of progesterone action. Ann N Y Acad Sci. 1977;286:408–420. doi: 10.1111/j.1749-6632.1977.tb29433.x. [DOI] [PubMed] [Google Scholar]
- Schuetz AW, Cloud JG. Steroid-Cell Surface Interactions in the Induction of Meiotic Maturation in Amphibian Oocytes: Method for and Effect of Local Application of Progesterone. Differentiation. 1977;8:191–194. doi: 10.1111/j.1432-0436.1977.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Browne R, Seratt J, Vance C, Kouba A. Hormonal priming, induction of ovulation and in-vitro fertilization of the endangered Wyoming toad (Bufo baxteri) Reprod Biol Endocrinol. 2006;4:34. doi: 10.1186/1477-7827-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau VL, Somoza GM, Natale GS, Pauli B, Wignall J, Jackman P, Doe K, Schueler FW. Hormonal induction of spawning in 4 species of frogs by coinjection with a gonadotropin-releasing hormone agonist and a dopamine antagonist. Reprod Biol Endocrinol. 2010;8:36. doi: 10.1186/1477-7827-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouba AJ, Vance CK. Applied reproductive technologies and genetic resource banking for amphibian conservation. Reprod Fertil Dev. 2009;21:719–737. doi: 10.1071/RD09038. [DOI] [PubMed] [Google Scholar]
- Johnson CJ, Vance CK, Roth TL, Kouba AJ. Oviposition and ultrasound monitoring of American toads (Bufo americanus) treated with exogenous hormones. Proc Am Assoc Zoo Vet. 2002. pp. 299–301.
- Browne R, Li H, Seratt J, Kouba A. Progesterone improves the number and quality of hormone induced Fowler toad (Bufo fowleri) oocytes. Reprod Biol Endocrinol. 2006;4:3. doi: 10.1186/1477-7827-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael S, Buckley C, Toro E, Estrada A, Vincent S. Induced ovulation and egg deposition in the direct developing anuran Eleutherodactylus coqui. Reprod Biol Endocrinol. 2004;2:6. doi: 10.1186/1477-7827-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne R, Gaikhorst G, Vitali S, Roberts JD, Matson P. Exogenous hormones induce poor rates of oviposition in the anurans, Litoria moorei and L. aurea. App Herpetol. 2008;5:81–86. doi: 10.1163/157075408783489194. [DOI] [Google Scholar]
- Mann RM, Hyne RV, Choung CB. Hormonal induction of spermiation, courting behavior and spawning in the southern bell frog, Litoria raniformis. Zoo Biol. 2010;29:774–782. doi: 10.1002/zoo.20331. [DOI] [PubMed] [Google Scholar]
- Dubois A. New proposals for naming lower-ranked taxa within the frame of the International Code of Zoological Nomenclature. C R Biol. 2006;329:823–840. doi: 10.1016/j.crvi.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Houlahan JE, Findlay CS, Schmidt BR, Meyer AH, Kuzmin SL. Quantitative evidence for global amphibian population declines. Nature. 2000;404:752–755. doi: 10.1038/35008052. [DOI] [PubMed] [Google Scholar]
- Stuart S, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fishman DL, Waller RW. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- Wake DB. Declining amphibian populations. Science. 1991;253:860. doi: 10.1126/science.253.5022.860. [DOI] [PubMed] [Google Scholar]
- Tyler MJ, Knight F. Field guide to the frogs of Australia. Collingwood: CSIRO Publishing; 2009. [Google Scholar]
- Amaya E, Offield MF, Grainger RM. Frog genetics: Xenopus tropicalis jumps into the future. Trends Genet. 1998;14:253–255. doi: 10.1016/S0168-9525(98)01506-6. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. FSH stimulates hyaluronic acid synthesis by oocyte-cumulus cell complexes from mouse preovulatory follicles. Nature. 1979;281:483–484. doi: 10.1038/281483a0. [DOI] [PubMed] [Google Scholar]
- Su Y-Q, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Mitogen-Activated Protein Kinase Activity in Cumulus Cells Is Essential for Gonadotropin-Induced Oocyte Meiotic Resumption and Cumulus Expansion in the Mouse. Endocrinology. 2002;143:2221–2232. doi: 10.1210/en.143.6.2221. [DOI] [PubMed] [Google Scholar]
- Fortune J. Steroid production by Xenopus ovarian follicles at different developmental stages. Dev Biol. 1983;99:502–509. doi: 10.1016/0012-1606(83)90299-3. [DOI] [PubMed] [Google Scholar]
- Greenwald GS, Choudary JB. Follicular Development and Induction of Ovulation in the Pregnant Mouse. Endocrinology. 1969;84:1512–1516. doi: 10.1210/endo-84-6-1512. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, Murray AA, Simmer G, Hufford MG, Smith MF, Spears N, Lubahn DB. Gonadotropin Induction of Ovulation and Corpus Luteum Formation in Young Estrogen Receptor-Î ± Knockout Mice. Biol Reprod. 2000;62:599–605. doi: 10.1095/biolreprod62.3.599. [DOI] [PubMed] [Google Scholar]
- Wright PJ. The induction of oestrus and ovulation in the bitch using pregnant mare serum gonadotrophin and human chorionic gonadotrophin. Aust Vet J. 1980;56:137–140. doi: 10.1111/j.1751-0813.1980.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Batta SK, Stark RA, Brackett BG. Ovulation Induction by Gonadotropin and Prostaglandin Treatments of Rhesus Monkeys and Observations of the Ova. Biol Reprod. 1978;18:264–278. doi: 10.1095/biolreprod18.2.264. [DOI] [PubMed] [Google Scholar]
- Howard JG, Roth TL, Byers AP, Swanson WF, Wildt DE. Sensitivity to exogenous gonadotropins for ovulation induction and laparoscopic artificial insemination in the cheetah and clouded leopard. Biol Reprod. 1997;56:1059–1068. doi: 10.1095/biolreprod56.4.1059. [DOI] [PubMed] [Google Scholar]
- Moore NW. The control of time of oestrus and ovulation and the induction of superovulation in cattle. Aust J Agric Res. 1975;26:295–304. doi: 10.1071/AR9750295. [DOI] [Google Scholar]
- Turman EJ, Laster DB, Renbarger RE, Stephens DF. Multiple Births in Beef Cows Treated with Equine Gonadotropin (PMS) and Chorionic Gonadotropin (HCG) J Anim Sci. 1971;32:962–967. doi: 10.2527/jas1971.325962x. [DOI] [PubMed] [Google Scholar]
- Duchamp G, Bour B, Combarnous Y, Palmer E. Alternative solutions to hCG induction of ovulation in the mare. J Reprod Fertil Suppl. 1987;35:221–228. [PubMed] [Google Scholar]
- Harrison LA, Squires EL, McKinnon AO. Comparison of HCG, buserelin and luprostiol for induction of ovulation in cycling mares. J Equine Vet Sci. 1991;11:163–166. doi: 10.1016/S0737-0806(07)80039-6. [DOI] [Google Scholar]
- Omata S. Relative roles of jelly layers in successful fertilization of Bufo japonicus. J Exp Zool. 1993;265:329–335. doi: 10.1002/jez.1402650315. [DOI] [Google Scholar]
- Elinson RP. A block to cross-fertilization located in the egg jelly of the frog Rana clamitans. J Embryol Exp Morphol. 1974;32:325–335. [PubMed] [Google Scholar]
- Subcommittee on Amphibian Standards CoS, Institute of Laboratory Animal Resources, National Research Council. Book Amphibians. Guidelines for the breeding, care, and management of laboratory animals. National Academy of Sciences; 1974. Amphibians. Guidelines for the breeding, care, and management of laboratory animals. [Google Scholar]
- Frost DR, Grant T, Faivovich J, Bain R, Haas A, Haddad CFB, de Sá RO, Donnellan SC, Raxworthy CJ, Wilkinson M. et al. The amphibian tree of life. Bull Am Mus Nat Hist. 2006;297:1–370. [Google Scholar]
- Silla A. Effect of priming injections of luteinizing hormone-releasing hormone on spermiation and ovulation in Gunther's toadlet, Pseudophryne guentheri. Reprod Biol Endocrinol. 2011;9:68. doi: 10.1186/1477-7827-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne P, Silla A. Hormonal induction of gamete release, and in-vitro fertilisation, in the critically endangered Southern Corroboree Frog, Pseudophryne corroboree. Reprod Biol Endocrinol. 2010;8:144. doi: 10.1186/1477-7827-8-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks CB, Birkett J, Young S, Vincent M, Hawkes T. Breeding and management of the Great Barred Frog, Mixophyes fasciolatus, at Melbourne Zoo. Herpetofauna. 2003;33:2–12. [Google Scholar]