Abstract
Numerous challenges remain in the successful clinical translation of cell-based therapeutic studies for skeletal tissue repair, including appropriate cell sources and viable cell delivery systems. Poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL) amphiphilic block copolymers have been extensively explored in microspheres preparation. Due to the introduction of hydrophilic PEG segments into PCL backbones, these copolymers have shown much more potentials in carrying protein, lipophilic drugs or genes than commonly used poly (ε-caprolactone) (PCL) and poly (lactic acid). The aim of this study is to investigate the attachment and osteogenic differentiation of human placenta derived mesenchymal stem cells (PMSCs) on PEG-PCL triblock copolymers nanofiber scaffolds. Here we demonstrated that PMSCs proliferate robustly and can be effectively differentiated into osteogenic-like cells on nanofiber scaffolds. This study provides evidence for the use of nanofiber scaffolds as an ideal supporting material for in vitro PMSCs culture and an in vivo cell delivery vehicle for bone repair.
Keywords: Placenta derived human mesenchymal stem cells (PMSCs), Osteogenic differentiation, Electrospun nanofiber scaffolds
Introduction
Bone is one of the few tissues in the body that undergoes true regeneration in response to injury, and many of the mechanisms involved in skeletal repair appear to recapitulate the events of embryologic development (Ferguson et al. 1999; Einhorn and Lee 2001; Gerstenfeld et al. 2003b). Tissue engineering strategies that deliver cells and growth factors on scaffolds have demonstrated considerable potential in developing bone graft substitutes. However, limits still exist in the cell-based therapy for bone regeneration, in part, due to the inaccessibility of adequate osteogenic cells as well as effective cell delivery systems (Kolambkar et al. 2010).
To reconstruct damaged bone tissues, the identification of cell sources that can be easily harvested, expanded and controllably differentiated is of vital importance. Mesenchymal stem cells (MSCs) as a potential source of stem cells are widely used in hematopoietic recovery, regenerative medicine and tissue engineering (Brooke et al. 2007, 2009; Pittenger et al. 1999; Studeny et al. 2002; Chen et al. 2006). It has been demonstrated that bone-marrow-derived mesenchymal stem cells (BMSCs) are the most commonly used cell type in bone regeneration and autologous transplantation, thus BMSCs are quite promising in bone tissue engineering approaches. However, bone marrow is rare in a large volume and the differentiation potential of BMSCs significantly decreased with age (Mareschi et al. 2006; Pittenger et al. 1999; Fehrer and Lepperdinger 2005). Until novel technology can be applied, the collection of bone marrow still utilizes invasive procedures (Suva et al. 2004; Nosanchuk et al. 1996). Placental tissues have immunomodulatory properties in maintaining fetomaternal tolerance during pregnancy, as a suitable candidate source of MSCs, placenta derived mesenchymal stem cells (PMSCs) may represent a pool of stem cells (Parolini et al. 2008). More attractively, placenta tissues can be easily obtained since they are discarded after the delivery of babies (Evangelista et al. 2008; Brooke et al. 2007, 2009).
Extracellular matrix (ECM) in natural tissues can support cell attachment, proliferation, and differentiation. Ideally biomimetic scaffold should mimic natural ECM as much as possible. Over the past decades, the use of electrospun in biomedical applications increased drastically (Dzenis 2004; Liao et al. 2006; Shin et al. 2006). When used as scaffolds, electrospinning has gained rising popularity as a means of fabricating scaffolds with micro to nanoscale features similar to the hierarchical structure of the native ECM. The nanofibrous scaffold also plays a critical role in providing the appropriate chemical, morphological and structural cues to direct the MSCs towards a targeted functional outcome (Li et al. 2003; Venugopal and Ramakrishna 2005; Yoshimoto et al. 2003; Prabhakaran et al. 2009).
Poly (ε-caprolactone) (PCL) is hydrolytic and cleared by the US Food and Drug Administration for internal use in the human body. A wide variety of tissue engineering applications have demonstrated the ability of PCL to form cell-scaffold complexes in vivo (Quynh et al. 2006; Tuzlakoglu et al. 2005). As a typical hydrophilic polymer, PEG is also approved for clinical use by the Food and Drug Administration (FDA). Low molecular weight PEG is readily excreted through kidney (Choi and Kim 2002; Cotí et al.2009). Previous studies have shown triblock copolymer poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCL-PEG-PCL, PCEC) has higher degradation rate, hydrophilicity but lower acidity of the derivative products compared to pure PCL, thus exhibiting a good potential in forming cell-scaffold complexes (Zhou et al. 2003; Xie et al. 2010). When used as protein and peptide carrier or gene delivery cargo, PCEC microspheres are more ideal than other commonly used PCL and PLA materials, due to the introduction of hydrophilic PEG segments into PCL backbones (Bakandritsos et al. 2010; Zhou et al. 2003). Till now, knowledge is still lacking in understanding the colonization and osteogenic differentiation of PMSCs on PCEC scaffold. Therefore, this study is to gain knowledge of osteogenic differentiation of human PMSCs on PCEC nanofiber scaffold.
Materials and methods
Isolation and culture of PMSCs
Human placentas were procured from healthy donor mothers during routine caesarean section births with full informed consent. The placentas were transported to the National Key Laboratory of Biotherapy and Cancer Center, placenta tissues were cut into 1–2 mm pieces and the blood cells were washed in PBS, then the tissue pieces were kept in 50 ml tube (BD, Franklin Lakes/ NJ, USA) and digested with 1 mg/ml collagenase II (Sigma, St, Louis/Mo, USA) for 2 h at 37 °C in an incubated shaker. Then the cells were washed for another 2 times in Dulbecco’s modified Eagle’s medium–low glucose (DMEM–LG) (Gibco, Grand Island/NY, USA) and cultured in DMEM–LG with 10 % FBS (Gibco, Mulgrave Victoria, Australia), 1 % penicillin/streptomycin (Gibco, Australia) at 37 °C under an atmosphere of 5 % CO2 in humidified air.
Cell surface phenotype
The plate-adhering population of cells (5 × 105) was trypsinized and incubated with mouse anti-human monoclonal antibodies [CD29, CD44, CD45, CD105 and CD166] (Thermo, Millipore, eBioscience, Neomarkers, Invitrogen) for 30 min at 37 °C in PBS. FITC-conjugated anti-mouse secondary antibody was added for 30 min without light at 37 °C in 100 μl PBS. The same FITC labeled mouse IgG (BD Biosciences, USA) without primary antibodies served as negative controls.
Preparation of 3D nanofiber scaffold
Poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCL-PEG-PCL, PCEC) copolymer was synthesized by ring-opening polymerization as described previously (Zhou et al. 2003). According to Gel Permeation Chromatography (GPC) result, the average molecular weight (Mw) was 1.02 × 105 g/mol, polydispersity (PDI) was 2.10. PCEC copolymers were dissolved in dichloromethane to prepare an 8 wt% solution. The polymer solution was loaded into a 20 ml syringe, placed in a syringe pump (model WZ-50C66T, Smiths Medical Instrument (Zhejiang Co., Ltd, Hangzhou, China)), and the syringe was connected to a blunt-tipped 5-gauge needle. The extrusion flow rate was from 3 ml/h. The needle was connected to DC voltage power supply (Beijing Machinery & Electricity Institute, Beijing, China) at 18 kV. The grounded collector was positioned at a distance of 12 cm from the tip of the needle and consisted of an aluminum foil 10 cm × 10 cm in cross section. The recovered samples were placed in a vacuum oven at room temperature to fully eliminate the solvent for at least 48 h. All experiments with respect to the preparation of the scaffolds were carried out at 23 ± 2 °C, and the environmental humidity was controlled between 45 and 50 %. The fiber mats were sterilized with ethylene oxide (ETO) steam for 24 h at 37 °C before using for in vitro and in vivo studies.
Observation of scanning electron microscope
The morphology of the electrospun ultrafine fibers was investigated by a field emission Scanning Electron Microscope (SEM; Inspect F, FEI, Eindhoven, The Netherlands) and observed at 20 kV accelerating voltage. Before the observation, the samples were directly coated with gold using a sputter coater (KYKY SBC-12 Ion Sputtering Coater, KYKY Technology Development Ltd, Beijing, China).
Morphology of PMSCs cultured on three-dimensional porous scaffolds
The morphology of PMSCs seeded on PCEC scaffolds was observed by SEM and fluorescence microscopy. Briefly, the cells were trypsinized and seeded at a density of 2 × 105 on three-dimensional porous scaffolds, which were cut into a round shape and disinfected by ethylene oxide at 37 °C for 12 h in 6-well plate (Corning, Lowell/MA, USA). For SEM observation, cells were rinsed with PBS twice after 2 days of culture, then fixed in 3 % glutaraldehyde for about 2 h and post-fixed with 1 % OsO4 for 15 min. The samples were dehydrated through a series of graded concentration of ethanol (30, 50, 70, 80, 90, 95, and 100 %). Subsequently the samples were treated twice with hexamethyldisilazane (HMDS; Kelong Chemicals, Chengdu, China) each for 20 min and kept in a fume hood for air drying. Finally the sample surfaces were coated with a thin layer of gold using a Sputter Coater System and observed by SEM. For fluorescence microscopy observation, PMSCs seeded on PCEC scaffolds were stained with the fluorescent dye CM-DiI (Invitrogen, Carlsbad/CA, USA) for 20 min at 37 °C, and then stained with coumarin (Sigma, USA) for 5 min at 37 °C. The cells were washed with PBS for 3 times and observed with an invert microscope immediately. PMSCs seeded on 6-well plate were stained with the CM-DiI as control.
Proliferation of PMSCs cultured on PCEC nanofiber scaffolds
The proliferation of PMSCs on PCEC scaffolds was determined using MTT (3-{4,5-dimethylthiazol-2yl}-2,5-diphenyl-2H-tetrazolium-bromide) assay (Roche Diagnostics, Mannheim, Germany) after induction by osteogenic differentiation medium on days 1, 3, 7, and 14. Briefly, PCEC scaffolds (diameter 5 mm) were put in each well of the 96-well plate (Corning, USA) and sterilized by ethylene oxide as described above. 2 × 103 PMSCs in a final volume of 100 μl per well were seeded on the scaffolds and the cells directly seeded on 96-well plate (Corning, USA) were used as control. 24 h later, the DMEM–LG medium was changed to osteogenic induction medium. 10 μl of MTT labeling regent (5 mg/ml) was added to each well at the predetermined time and cells were cultured for an additional 4 h, then precipitated formazan was dissolved in 100 μl solubilization solution overnight. In order to release all of the color, the scaffolds were washed completely in solubilization solution. 100 μl supernatant was transferred into a new 96 well plate and the optical density (OD) at 490 nm was measured with spectrophotometer (Spectronic™ 20D+; Thermo Fisher Scientific, Shanghai, China). All experiments were performed in triplicate.
Alizarin red staining
2 × 105 cell suspension was added on PCEC nanofibers that had been placed into 6-well plate, PMSCs seeded directly on 6-well plate (Corning, USA) were used as control. 24 h later, DMEM–LG medium was changed to osteogenic differentiation medium which contains 0.1 μM dexamethasone, 10 mM β-glycerol phosphate, and 50 μg/ml ascorbate-2 (Sigma, USA) in Dulbecco’s modified Eagle’s medium–high glucose (DMEM–HG) (Gibco, USA) medium with 10 % FBS (Gibco, Australia). The medium was changed twice a week. 2 weeks later, the cells were gently washed with PBS, fixed with 10 % formalin for 15 min and stained with Alizarin red solution (Sigma, USA) for 30 min. Calcium deposits were checked microscopically for an orange-red color.
Western blot analysis
PMSCs were cultured on PCEC nanofibers and incubated with osteogenic differentiation medium as described above. PMSCs seeded into 6-well plate (Corning, USA) or PCEC nanofibers treated with DMEM–HG medium were used as negative controls, and PMSCs seeded into 6-well plate treated with osteogenic differentiation medium were used as positive control. After 2 weeks of cultivation, the cells were harvested and lysed with protein extraction reagent (Thermo Fisher Scientific, Waltham/MA, USA) according to the previously reported method (Benzinger et al. 2005). Protein content was quantified by Bradford assay method (GenMed, Arlington/MA, USA). Equal amounts of proteins were applied to and separated by SDS–polyacrylamide gels electrophoresis and transferred to polyvinylidene difluoride membrane (Millipore, Billerica/MA, USA). Polyclonal antibodies against osteocalcin (OC) and osteopontin (OPN) (Abcam, Cambridge, UK) were added at a 1:1000 dilution and incubated overnight. β-actin was used to normalize data. Bands were analyzed using the enhanced chemiluminescence detection system and exposed to Kodak BioMax X-ray film.
Alkaline phosphatase assay
The alkaline phosphatase (ALP) activity of PMSCs cultured on scaffolds was measured according to the provided protocol. The PMSCs/scaffold constructs seeded for 7 and 14 days were collected and sonicated into ice in protein extraction buffer. PMSCs seeded into 6-well plate were used as a positive control (Corning, USA). Cell lysates at the same concentration were incubated with p-nitrophenyl phosphate solution (16 mM, Sigma Chemical, St. Louis/MO, USA) at 37 °C for 30 min. ALP activity was normalized by total protein content of the samples. The production of p-nitrophenol in the presence of ALP was measured by monitoring light absorbance at 405 nm. Each assay was performed in triplicate and the assays were repeated for three times.
Statistical analysis
All experiments in this study were performed in at least three independent replicates. The statistical differences between the experimental groups were evaluated by Student’s t test; differences were considered significant when p values were below 0.05.
Results
Characterization of isolated PMSCs
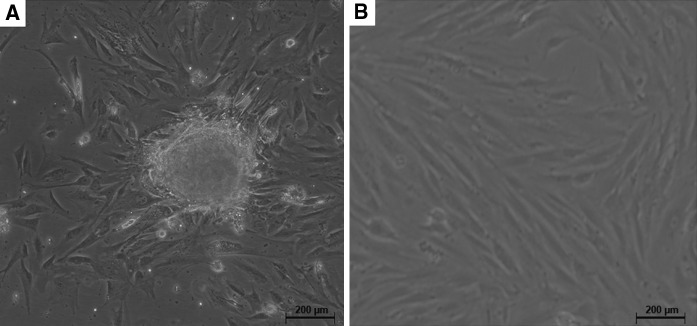
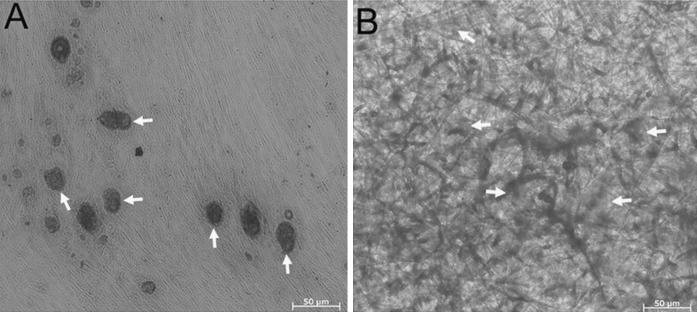
In this study, the culture of PMSCs was initiated by cultivation of collagenase II digested placenta. After 48 h of incubation, the fibroblast-like cells began to migrate-out from the placentas, and after a 72 h culture period colonies could be observed (Fig. 1a). After culturing for 2 passages, classical fibroblast-like or vortex-shaped structure could be observed (Fig. 1b). Only the cells before 5 population doubling were use in the experiments.
Fig. 1.
Morphology of placenta derived mesenchymal stem cells (PMSCs). a After initial culturing for 72 h, fibroblast-like cells migrated out from the enzyme-digested placenta tissues and fibroblastic colony formation could be observed Scale bar 200 μm. b Cells prominently displayed a fibroblast-like or vortex-shaped morphology after 2 passages of cultivation. Scale bar 200 μm
Cell surface phenotype
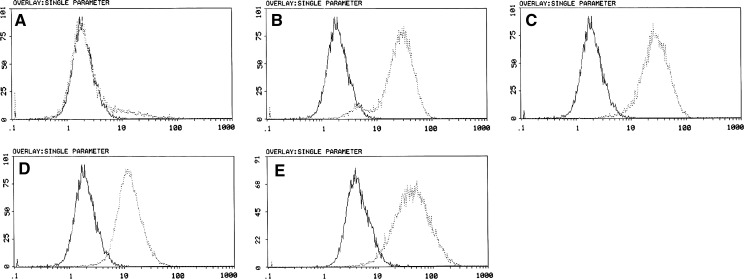
Flow cytometric phenotype showed that PMSCs were positive for CD29, CD44, CD105, CD166, and negative for hematopoietic markers CD45 (Fig. 2).
Fig. 2.
Immunophenotypic characterization of PMSCs detected by flow cytometry (FCM). Cells were labeled with specific monoclonal antibodies for mesenchymal markers (dotted line) or isotype controls (solid line). The isolated PMSCs were positive for CD29 (b), CD44 (c), CD105 (d) and CD166 (e), but negative for hematopoietic marker CD45 (a) (Passage 5). The experiments were repeated at least three times, and similar results were observed
Morphology of three-dimensional nanofiber scaffolds
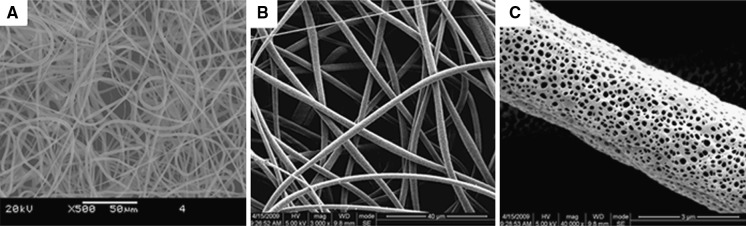
SEM of electrospun PCEC nanofiber revealed a 3D nonwoven network with a diameter of 2875 ± 445 nm (Fig. 3a, b). Fibers were porous (95 ± 45 nm) in structure (Fig. 3c).
Fig. 3.
Electrospun PCEC nanofibers characterization. a Mesh-like structure of PCEC under SEM; b higher SEM magnification of PCEC; c imaging of the individual fiber surface
Adhesion and growth of PMSCs cultured on three-dimensional nanofiber scaffolds
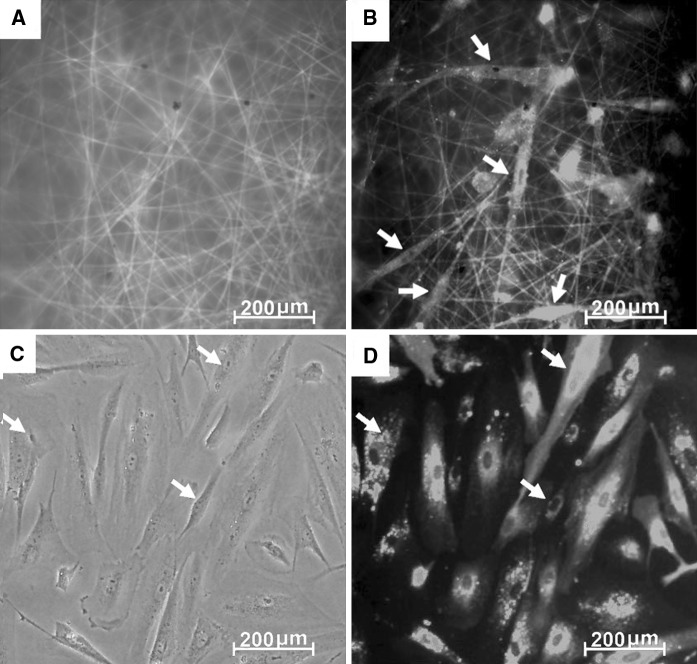
SEM was used to evaluate the morphology and biocompatibility of PMSCs cultured on PCEC scaffolds. As shown in Fig. 4, the predominant PMSCs covered the surface of the scaffolds and spread with a physiological adherence pattern. Fluorescence microscopic images were taken for further study the cytocompatibility of PCEC nanofiber scaffolds for growing PMSCs (Fig. 5a). Fig. 5b showed that the cells were spread and maintained an elongated and fibroblast-like shape after 2 days of cultured on the scaffolds compared with that in 6-well plate (Fig. 5c, d). Our study demonstrated that the hydrophilic but less acid PCEC nanofiber scaffolds were suitable for the adhesion of PMSCs.
Fig. 4.

SEM micrographs of PMSCs (black arrow) growing on nanofibers after 2 days culture. Scale bar 100 μm
Fig. 5.
Fluorescent micrographs of PMSCs cultured on PCEC nanofibrillar surfaces. a Green fluorescence images of coumarin-stained PCEC scaffolds. Scale bar 200 μm. b Red fluorescence images of CM-Dil stained PMSCs (white arrows) cultured on PCEC scaffolds; scale bar 200 μm. c PMSCs (white arrows) cultured in 6-well plate. Scale bar 200 μm. d Red fluorescence images of CM-Dil stained PMSCs (white arrows) cultured in 6-well plate. Scale bar 200 μm
Proliferation of PMSCs cultured on PCEC nanofiber scaffold
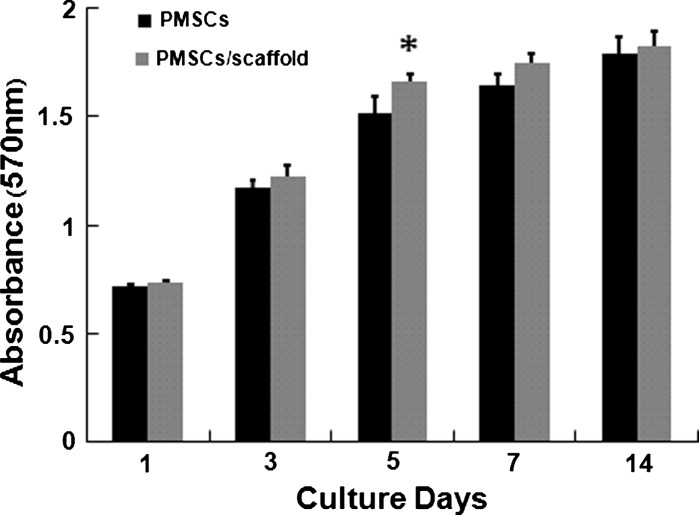
As the statistical analysis data showed (Fig. 6) there was no significant difference in the initial proliferation rate (day 1, 3) when cells were cultured under differentiation conditions. After 5 days of culture, mean cell proliferation rates of PMSCs on PCEC nanofiber scaffolds were significantly higher compared to cells growth in 96-well plate (Fig. 6). Nevertheless, 7 days later this effect was not significant any more, and PMSCs cultured on scaffolds or 96-well plate had the same relative cell densities after 2 weeks of cultivation. This demonstrated that PCEC nanofiber had little impact on PMSCs proliferation, which is important for the application of biomaterial nanofiber scaffolds in regenerative medicine and tissue engineering.
Fig. 6.
MTT assay for assessing the proliferation of PMSCs cultured on PCEC scaffolds on days 1, 3, 5, 7, and 14, with the PMSCs cultured in tissue culture plate as control. Error bars represent means ± SD for n = 3, Significant differences were confirmed statistically: Student’s t test, * p < 0.05
Differentiation of PMSCs into osteogenic cells on PCEC nanofiber scaffolds
To evaluate the osteogenic differentiation of PMSCs on PCEC nanofiber scaffold, the cells were cultured with induction factors as described above. Alizarin red staining was used to qualitatively confirm calcium deposition. As shown in Fig. 7, 2 weeks’ incubation of PMSCs on PCEC nanofibers resulted in an increase of mineral depositions, which was similar to that on 6-well plate. These data demonstrated that, the PCEC nanofiber scaffolds had no observable negative influence on osteoblast differentiation of PMSCs.
Fig. 7.
Alizarin Red staining. a PMSCs (white arrows) seeded in 6-well plate and treated with osteogenic differentiation medium as positive control. Scale bar 50 μm. b PMSCs (white arrows) seeded on PCEC nanofibers and treated with osteogenic differentiation medium showed positive staining to Alizarin Red S after 2 weeks of culture. Scale bar 50 μm
Western blot analysis
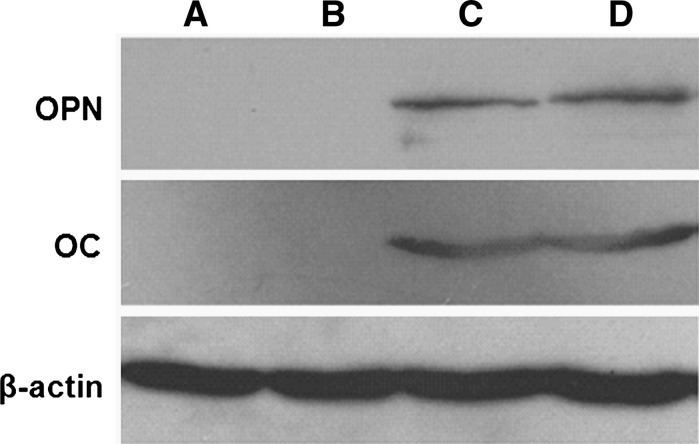
As shown in Fig. 8, PMSCs cultured on PCEC nanofibers showed no significant difference from PMSCs cultured on 6-well plate in osteogenic differentiation medium, but PMSCs cultured on PCEC nanofibers with osteogenic induction medium had significantly increased the expression of OPN and OC compared to those cultured with DMEM–HG. This result showed that PCEC nanofiber scaffolds have no negative influence on the osteogenic differentiation of PMSCs, which indicated that PCEC nanofiber scaffolds can be used as carriers for PMSCs culture and delivery systems.
Fig. 8.
Western blotting analysis of osteocalcin and osteopontin expression for PMSCs cultured on PCEC nanofiber scaffolds. a PMSCs seeded in 6-well plate and treated with DMEM–HG with 10 % FBS as negative control; b PMSCs seeded on PCEC nanofibers and treated with DMEM–HG with 10 % FBS; c PMSCs seeded in 6-well plate and treated with osteogenic differentiation medium as positive control; d PMSCs seeded on PCEC nanofibers and treated with osteogenic differentiation medium
Alkaline phosphatase activity
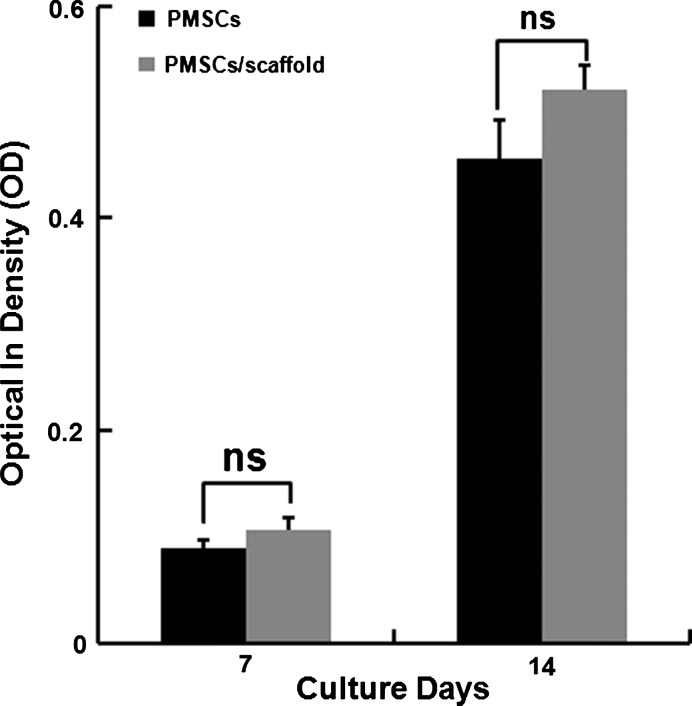
PMSCs seeded into 6-well plate were used as positive control. For performing the alkaline phospatase activity tests, the same concentration of cell lysates was incubated with p-nitrophenyl phosphate solution (16 mM) at 37 °C for 30 min. The ALP activity of PMSCs cultured on PCEC nanofiber scaffolds increased from 0.1077 ± 0.0104 at 7 days of culture to 0.5203 ± 0.024 at 14 days of culture (Fig. 9). The levels of ALP activity did not show statistical significance compared with the cells cultured on 6-well plate on the 7th day (0.0906 ± 0.006, p = 0.074) on the 14th day (0.456 ± 0.038, p = 0.066). These results indicated that PCEC nanofiber scaffolds had no negative influence on the initiation of osteogenic differentiation of PMSCs.
Fig. 9.
ALP activity of PMSCs cultured on PCEC nanofiber scaffolds. Measurements correspond to ALP activity on days 7 (p = 0.074) and 14 (p = 0.066) (n = 3). Data represent means ± SD of three independent experiments. ns no significance
Discussion
With the development of regenerative medicine and tissue engineering, MSCs are increasingly used in pre-clinical and clinical studies (Uccelli et al. 2008). However, due to the low yield and the complicated invasive procedure of MSCs (Pederson and Parran 1999; Shamsul et al. 2004) the use of MSCs is hampered. PMSCs that are similar to BMSCs in cell-surface marker and multilineage differentiation potency but can be harvested much easier and at much higher numbers than BMSCs (Miao et al. 2006; Wulf et al. 2004) will undoubtedly play a key role in the development of regenerative medicine.
Our previous studies have found that PCEC microspheres showed a higher potential as carriers for protein, peptide and gene delivery systems than the commonly used PCL and PLA due to the introduction of hydrophilic PEG segments into the PCL backbones (Gou et al. 2009). In the present study, we demonstrated that PCEC nanofiber scaffolds can also be used as carriers for cell delivery systems. After 14 days of culture, PMSCs cultured on PCEC showed no significant difference compared with PMSCs cultured in 6-well plate in vitro cell growth and osteogenic differentiation. In particular, the ALP activity and the expression of the osteogenic marker OC, OPN for PMSCs cultured on PCEC nanofibers showed no significant difference with PMSCs cultured in 6-well plate. In addition, our preliminary data also show that PCEC nanofibers have a better potential for the attachment, colonization and differentiation of PMSCs compared with PCL and PLA (data not shown). Further studies should be carried out to test the proliferation and differentiation of PMSCs on PCEC absorbed with chemical compounds or proteins, such as growth factors.
In conclusion, in the present study we described a safe, reliable and high-yield protocol for isolation and culture of PMSCs from placenta, and for the first time we demonstrated that PMSCs are able to attach, colonize, and undergo robust osteogenic differentiation on PCEC electrospun nanofiber meshes. Further studies are needed to investigate the use of PMSCs cultured on PCEC nanofiber scaffolds as a cell delivery vehicle for the repair of bone defects in vivo.
Acknowledgments
This work was supported by the grants from the National Natural Sciences Foundation of China (30900768).
Contributor Information
Aiping Tong, Email: aipingtong@gmail.com.
Gang Guo, Email: guogang1999@sina.com.
References
- Bakandritsos A, Mattheolabakis G, Zboril R, Bouropoulos N, Tucek J, Fatouros DG, Avgoustakis K. Preparation, stability and cytocompatibility of agnetic/PLA-PEG hybrids. Nanoscale. 2010;2:564–572. doi: 10.1039/b9nr00253g. [DOI] [PubMed] [Google Scholar]
- Benzinger A, Muster N, Koch HB, Yates JR, Hermeking H. Targeted proteomic analysis of 14–3-3 sigma, a p53 effector commonly silenced in cancer. Mol Cell Proteomics. 2005;4:785–795. doi: 10.1074/mcp.M500021-MCP200. [DOI] [PubMed] [Google Scholar]
- Brooke G, Cook M, Blair C, Han R, Heazlewood AC, Jones B, Kambouris M, Kollar K, McTaggart S, Pelekanos R, Rice A, Rossetti T, Atkinson K. Therapeutic applications of mesenchymal stromal cells. Semin Cell Dev Biol. 2007;118:846–858. doi: 10.1016/j.semcdb.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Brooke G, Rossetti T, Pelekanos R, Ilic N, Murray P, Hancock S, Antonenas V, Huang G, Gottlieb D, Bradstock K, Atkinson K. Manufacturing of human placenta-derived mesenchymal stem cells for clinical trials. Br J Haematol. 2009;144:571–579. doi: 10.1111/j.1365-2141.2008.07492.x. [DOI] [PubMed] [Google Scholar]
- Chen XC, Wang R, Zhao X, Wei YQ, Hu M, Wang YS, Zhang XW, Zhang R, Zhang L, Yao B, Wang L, YQ ZengTT, Yang JL, Tian L, Kan B, Lin XJ, Lei S, Deng HX, Wen YJ, Mao YQ, Li J. Prophylaxis against carcinogenesis in three kinds of unestablished tumor models via IL12-gene-engineered MSCs. Carcinogenesis. 2006;27:2434–2441. doi: 10.1093/carcin/bgl069. [DOI] [PubMed] [Google Scholar]
- Choi KS, Kim DJ. Drug-releasing behavior of MPEG/PLA block copolymer micelles and solid particles controlled by component block length. J Appl Polym Sci. 2002;83:435–445. doi: 10.1002/app.10072. [DOI] [Google Scholar]
- Cotí KK, Belowich ME, Liong M, Ambrogio MW, Lau YA, Khatib HA, Zink JI, Khashab NM, Stoddart JF. Mechanised nanoparticles for drug delivery. Nanoscale. 2009;1:16–39. doi: 10.1039/b9nr00162j. [DOI] [PubMed] [Google Scholar]
- Dzenis Y. Spinning continuous fibers for nanotechnology. Science. 2004;304:1917–1919. doi: 10.1126/science.1099074. [DOI] [PubMed] [Google Scholar]
- Einhorn TA, Lee CA. Bone regeneration: new findings and potential clinical applications. J Am Acad Orthop Surg. 2001;9:157–165. doi: 10.5435/00124635-200105000-00002. [DOI] [PubMed] [Google Scholar]
- Evangelista M, Soncini M, Parolini O. Placenta-derived stem cells: new hope for cell therapy? Cytotechnology. 2008;58:33–42. doi: 10.1007/s10616-008-9162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005;40:926–930. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87:57–66. doi: 10.1016/S0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- Gou ML, Wu L, Yin QQ, Guo QF, Guo G, Liu J, Zhao X, Wei YQ, Qian ZY. Transdermal anaesthesia with lidocaine nano-formulation pretreated with low-frequency ultrasound in rats model. J Nanosci Nanotechnol. 2009;9:6360–6365. doi: 10.1166/jnn.2009.1343. [DOI] [PubMed] [Google Scholar]
- Kolambkar YM, Peister A, Ekaputra AK, Kolambkar YM, Peister A, Ekaputra AK, Hutmacher DW, Guldberg RE. Colonization and osteogenic differentiation of different stem cell sources on electrospun nanofiber meshes. Tissue Eng Part A. 2010;16:3219–3230. doi: 10.1089/ten.tea.2010.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res. 2003;67A:1105–1114. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- Liao S, Li BJ, Ma ZW, Wei H, Chan C, Ramakrishna S. Biomimetic electrospun for tissue regeneration. Biomed Mater. 2006;1:45–53. doi: 10.1088/1748-6041/1/3/R01. [DOI] [PubMed] [Google Scholar]
- Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M, Madon E, Fagioli F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006;97:744–754. doi: 10.1002/jcb.20681. [DOI] [PubMed] [Google Scholar]
- Miao ZN, Jin J, Chen L, Zhu JZ, Huang W, JD QianHG, Zhang XG. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Nosanchuk JD, Sepkowitz KA, Pearse RN, White MH, Nimer SD, Armstrong D. Infectious complications of autologous bone marrow and peripheral stem cell transplantation for refractory leukemia and lymphoma. Bone Marrow Transplant. 1996;18:355–359. [PubMed] [Google Scholar]
- Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC. Concise review: isolation and characterization of cells from human term placenta: stem cell outcome of the first international workshop on placenta derived stem cells. Stem cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- Pederson C, Parran L. Pain in adult recipients of blood or marrow transplant. Cancer Nurs. 1999;22:397–407. doi: 10.1097/00002820-199912000-00001. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prabhakaran MP, Venugopal JR, Ramakrishna S. Mesenchymal stem cell differentiation to neuronal cells on electrospun nanofibrous substrates for nerve tissue engineering. Biomaterials. 2009;30:4996–5003. doi: 10.1016/j.biomaterials.2009.05.057. [DOI] [PubMed] [Google Scholar]
- Quynh PP, Upma S, Antonios G. Mikos electrospun poly(ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- Shamsul BS, Aminuddin BS, Ng MH, Ruszymah BH. Age and gender effect on the growth of bone marrow stromal cells in vitro. Med J Malaysia. 2004;59:196–197. [PubMed] [Google Scholar]
- Shin HJ, Lee CH, Cho IH, Kim YJ, Lee YJ, Kim IA, Park KD, Yui N, Shin JW. Electrospun PLGA nanofiber scaffolds for articular cartilage reconstruction: mechanical stability, degradation and cellular responses under mechanical stimulation in vitro. J Biomater Sci Polym Ed. 2006;17:103–119. doi: 10.1163/156856206774879126. [DOI] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-γ delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- Suva D, Garavaglia G, Menetrey J, Chapuis B, Hoffmeyer P, Bernheim L, Kindler V. Non-hematopoietic human bone marrow contains long-lasting, pluripotential mesenchymal stem cells. J Cell Physiol. 2004;198:110–118. doi: 10.1002/jcp.10396. [DOI] [PubMed] [Google Scholar]
- Tuzlakoglu K, Bolgen N, Salgado AJ, Gomes ME, Piskin E, Reis RL. Nano and micro-fiber combined scaffolds: a new architecture for bone tissue engineering. J Mater Sci Mater Med. 2005;16:1099–1104. doi: 10.1007/s10856-005-4713-8. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Venugopal J, Ramakrishna S. Biocompatible nanofiber matrices for the engineering of a dermal substitute for skin regeneration. Tissue Eng. 2005;11:847–854. doi: 10.1089/ten.2005.11.847. [DOI] [PubMed] [Google Scholar]
- Wulf GG, Viereck V, Hemmerlein B, Haase D, Vehmeyer K, Pukrop T, Glass B, Emons G, Trümper L. Mesengenic progenitor cells derived from human placenta. Tissue Eng. 2004;10:1136–1147. doi: 10.1089/ten.2004.10.1136. [DOI] [PubMed] [Google Scholar]
- Xie J, MacEwan MR, Schwartz AG, Xia Y. Electrospun for neural tissue engineering. Nanoscale. 2010;2:35–44. doi: 10.1039/b9nr00243j. [DOI] [PubMed] [Google Scholar]
- Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/S0142-9612(02)00635-X. [DOI] [PubMed] [Google Scholar]
- Zhou SB, Deng XM, Yang H. Biodegradable poly(ε-caprolactone)-poly(ethylene glycol) block copolymers: characterization and their use as drug carriers for a controlled delivery system. Biomaterials. 2003;24:3563–3570. doi: 10.1016/S0142-9612(03)00207-2. [DOI] [PubMed] [Google Scholar]