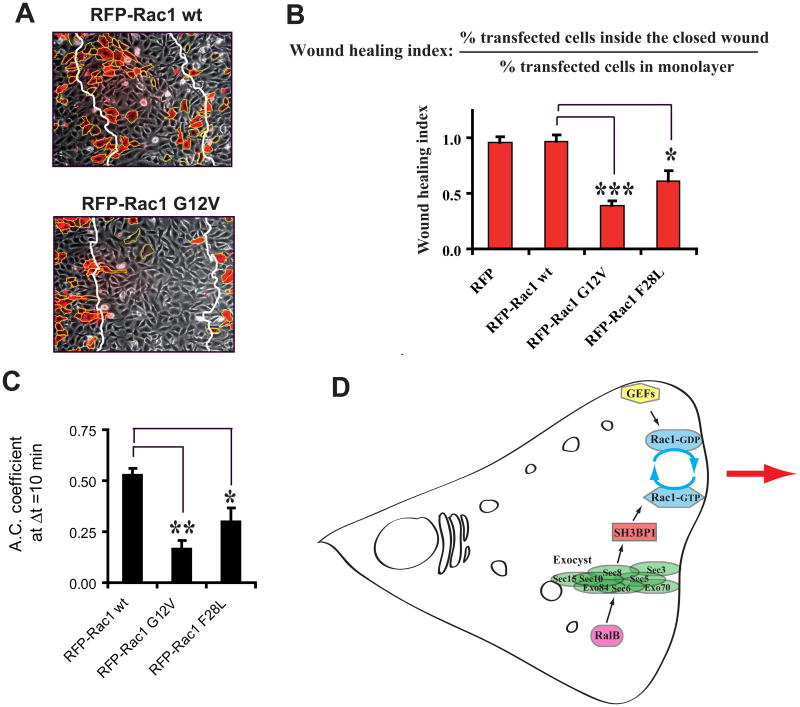
Figure 7. The Inactivation of Rac1 is a requirement for cell motility.
A. Expression of Rac1 GV12, but not of wild-type Rac1, inhibits cell migration in wound healing assay. NRK cells were transfected with vectors expressing RFP-fused Rac1 wild-type or carrying the G12V mutation. Representative fields showing the closed wound after migration are shown. Note that transfection efficiency was intentionally low.
B. Quantitative comparison of Rac1 alleles. The effects of the expression of three Rac1 alleles (wild-type, GTPase deficient G12V, fast-cycling F28L) on motility were quantified using the wound-healing index (see Figure S5C and Supplemental Experimental Procedures). Expression levels of RFP constructs were similar.
C. Temporal autocorrelation analysis. Effects of the expression of the three Rac1 alleles on morphodynamics were quantified as Figure 6I. See Movie S7 for representative videos. See Figure S5B for detailed temporal graphics of all analyzed cells.
D. A model for the interplay between Ral and Rac in the regulation of cell migration. In motile cells the RalB GTPase controls the association of the subunits of its effector the exocyst complex and promotes localization of the exocyst at leading edge. The exocyst physically interacts with and brings to the leading edge the GAP protein SH3BP1, which stimulates the hydrolysis of bound GTP to GDP on Rac1 at the front. Several GEFs activate Rac1 at the front by replacing bound GDP with GTP. Therefore, Rac1 undergoes locally multiple activation-inactivation cycles, and this Rac1 cycling is necessary for the spatiotemporal regulation of the protrusions during directional motility. RalB, via the exocyst, participates to define where Rac1 cycling occurs and promotes protruding activity.