Abstract
Objectives
Inflammatory markers such as high-sensitivity C-reactive protein (hsCRP) are associated with an increased risk of cardiovascular events and with the severity of peripheral arterial disease. The effects of inflammation on the development of vein graft disease remain speculative. We hypothesized that high levels of inflammatory markers would identify patients at increased risk for adverse events (graft failure, major cardiovascular events) after lower extremity bypass surgery.
Methods
Patients (n = 91) scheduled to undergo lower extremity bypass using autogenous vein were enrolled into a prospective study at two institutions. Exclusion criteria included the presence of major infection. A baseline plasma sample was obtained on the morning of lower extremity bypass. Biomarkers for inflammation included hsCRP, fibrinogen, and serum amyloid A (SAA). Values between patients with and without critical limb ischemia were compared. Proportions of events among dichotomized populations (upper limit of normal of each laboratory assay) were compared by log-rank test.
Results
Of the patients undergoing lower extremity bypass, 69% were men, 53% were diabetic, 81% were smokers, and their mean ankle-brachial index was 0.51 ± 0.19. The indication for lower extremity bypass was critical limb ischemia in 55%. There were no perioperative deaths and two early graft occlusions. During a mean follow-up of 342 days (range, 36–694 days) there were four deaths, 27 graft-related events, and 10 other cardiovascular events. No relationships were found between events and demographics, comorbidities, baseline ankle-brachial index, or statin use. High-sensitivity CRP (P = .005), fibrinogen (P < .001), and SAA (P = .0001) levels were associated with critical limb ischemia at presentation. Among patients with an elevated hsCRP (>5 mg/L) immediately before surgery, major postoperative vascular events occurred in 60% (21/35), compared with a 32% (18/56) rate in those with a baseline CRP <5 mg/L (P = .004, log-rank test). On multivariable analysis, only elevated hsCRP correlated with adverse graft-related or cardiovascular events (P = .018).
Conclusions
The inflammatory biomarkers of hsCRP, fibrinogen, and SAA correlate with peripheral arterial disease severity at presentation in patients undergoing lower extremity bypass. Patients with elevated hsCRP are at increased risk for postoperative vascular events, most of which are related to the vein graft. These findings suggest a potential relationship between inflammation and outcomes after lower extremity vein bypass surgery.
Approximately 10 million individuals have peripheral artery disease (PAD) in the United States, with a prevalence that approaches 10% in individuals aged >60 years.1 Among the most severely affected, nearly 100,000 peripheral artery reconstructions will be performed annually. Patients with PAD have coronary artery disease and cerebrovascular disease as comorbidities and have a mortality risk proportional to the severity of PAD.2 Atherosclerosis is an inflammatory disease3 with highly variable clinical progression. Hence, attention has turned to the use of inflammatory markers such as high-sensitivity C-reactive protein (hsCRP) for prognostic4–10 and potentially therapy-guiding purposes.4,11–13
Vein bypass surgery can provide effective and durable revascularization for patients with advanced PAD. However, vein graft failure occurs in 30% to 50% of patients ≤5 years,14,15 necessitating additional procedures and associated morbidity. Patient-specific risk factors remain poorly defined. Experimental studies demonstrate a prominent acute inflammatory response in the arterialized vein and have suggested that modulation of inflammation might influence the subsequent development of vein graft hyperplasia.16–19 Recent clinical observations, including reports of a possible beneficial effect of 3-hydroxy-3-methylglutaryl co-enzyme A (HMG CoA) reductase inhibitors (statins),20 lend further support to this concept. The role of inflammation in the incidence and progression of clinical vein graft disease remains highly speculative, however.
Beyond traditional lipid-fraction-based cardiovascular risk assessment, hsCRP has emerged as a predictive marker for myocardial infarction,4,21–23 stroke,4 and PAD,24,25 in apparently healthy men and women, thus associating chronic inflammation with these end points.26 Individuals with PAD have higher levels of hsCRP,27,28 and hsCRP also appears to be predictive of PAD progression as measured by both ankle-brachial index (ABI)29 and severity of functional impairment.30,31 Among individuals with PAD, hsCRP relates to both severity of disease on presentation and future cardiovascular events,27,32–34 including myocardial infarction in patients undergoing revascularization.34
Fibrinogen and serum amyloid A (SAA) are other circulating markers of inflammation in addition to hsCRP that are associated with cardiovascular disease (including PAD) and death.14,35–37 SAA is associated with the presence of coronary artery disease as well as with the development of cardiovascular complications in women presenting for coronary angiography.37 Fibrinogen levels are higher in subjects with PAD26,28 and are predictive of adverse cardiovascular events in this population.35
We hypothesized that inflammation is directly related to the development of vein graft disease as well as to the global atherosclerosis risk within the PAD population requiring surgical therapy. The overall goals of our investigation were to examine the relationships between circulating markers of inflammation, clinical events, and patterns of vein graft remodeling in patients undergoing infrainguinal bypass surgery for advanced PAD.
METHODS
Study design and population
This prospective cohort study examined the relationship between inflammatory markers and cardiovascular outcomes in patients undergoing lower extremity bypass with autogenous vein grafts. This study was undertaken at two Boston-area teaching hospitals and was approved by their respective Institutional Review Boards. Study enrollment began in February 2004 and was closed for this particular analysis in December 2005.
Patients were excluded if a nonautogenous conduit (in whole or in part) was used in their infrainguinal reconstruction, if they were unlikely to fully comply with the follow-up protocol (eg, long distance travel), or if they were unable to provide informed consent. Patients were also excluded if they had clinical evidence of infection including cellulitis, osteomyelitis, or deep space infection of the foot, or if they required operative débridement for sepsis control before bypass grafting. Other exclusion criteria included the use of immunosuppressive medications, concurrent systemic infections (eg, pneumonia, genitourinary tract infections), acute illness such as myocardial infarction or stroke, or any major surgery ≤30 days. All participants in this study provided written informed consent, and the protocol was approved by the Institutional Review Board of the Brigham and Women’s Hospital (Boston, Mass).
Blood collection and assays for inflammatory markers
Plasma and blood were collected on the morning of the subject’s index lower extremity bypass surgery by direct femoral vein puncture after the patient was anesthetized and before any surgical incision. Blood was collected into ethylenediaminetetraacetic acid and citrate Vacutainer tubes (BD Diagnostics, Franklin Lakes, NJ) and immediately iced. Tubes were spun at 3000 rpm for 20 minutes at 4°C in a refrigerated centrifuge. Baseline blood samples of the participants were stored at −70°C until the time of analysis. Assays for hsCRP, SAA, and fibrinogen were performed in a core laboratory using validated, high-sensitivity assays, as previously described.38 All marker assays were performed as a single batch at the conclusion of the study.
Operative procedures and clinical follow-up
Patients underwent autogenous lower extremity revascularization using standard techniques under the direction of the attending vascular surgeon, with either regional or general anesthesia. Five patients were subsequently excluded from the study because poor quality vein at the time of surgery required the use of a nonautogenous graft.
Details of the operative procedure were recorded, including the nature of the reconstruction, the need for adjunctive procedures, and the results of completion imaging studies. It is the standard practice of all participating surgeons to perform duplex ultrasound or completion angiogram, or both, as an intraoperative completion study. Any flow disturbances or intrinsic vein disease would be identified at this time. Preoperative and postoperative medical therapies, including the use of antithrombotics, were at the discretion of the attending surgeon and were recorded.
All patients were monitored for postoperative complications during their initial hospitalization and at all subsequent visits. All patients enrolled were followed up postoperatively by the attending vascular surgeon and received duplex ultrasound imaging surveillance at 1, 3, 6, 9, and 12 months, which is consistent with our standard practice. The duplex ultrasound examination was performed in an accredited vascular laboratory by a registered vascular technician.
After 12 months, the follow-up visits occurred every 6 months. All patients underwent a complete vascular examination at these follow-up time points and were evaluated for clinical and graft-related events and changes in their medication usage. In addition to the scheduled follow-up visits, two research coordinators, and a clinical research fellow (C. D. O.) monitored events through contact with the patients, their primary care providers, and the electronic medical record.
Definition of end points
Graft-related events included any graft revision (patch angioplasty or interposition graft), any percutaneous intervention to the bypass (balloon angioplasty), any graft occlusion (either salvaged or abandoned), or amputation in the index leg. Because four patients were identified near the closing of the study to have high-grade stenosis on graft surveillance (peak systolic velocity >300 cm/s or velocity ratio >3:1) but had not yet undergone revision, stenosis was included as a graft-related end point.
Contralateral limb events were any peripheral revascularization of the limb contralateral to the index limb, including percutaneous transluminal angioplasty/stent, open endarterectomy, or open revascularization with a bypass graft, as well as any major amputation.
Major cardiovascular events included stroke, myocardial infarction, cardiac revascularization procedure, or death from any cause. Myocardial infarction was documented by the presence of new Q waves with or without changes in the ST segments by standard 12-lead electrocardiograms and by specific enzyme elevations. All patients with myocardial infarction were seen by the cardiovascular medical service and the event was documented. A stroke was diagnosed by clinical evidence of focal neurologic deficit lasting >24 hours and correlated with an imaging modality such as computed tomography or magnetic resonance imaging. All strokes were documented by a neurologic consultant. An attempt was made at each death to determine the cause, but this was not possible in all circumstances because of refusal to consent for postmortem examination.
The primary end point of this study was a composite incidence of death, graft-related events, contralateral limb events, or other major cardiovascular events as defined.
Statistical methods
Student’s t test or χ2 was used to evaluate differences in means and proportions between patients with events and those without. The distribution of all inflammatory markers was non-Gaussian (rightward skewed) and is presented as median and interquartile range. Plasma levels of markers and bivariate relationships were analyzed by the one-way nonparametric analysis of variance (Kruskal-Wallis test) or the two-sample Wilcoxon rank sum (Mann-Whitney) test, where appropriate. Inter-relations between individual inflammatory markers were determined by linear regression of their natural log-transformations, as has been previously described.38 Correlations between inflammatory markers and collected parameters were evaluated with the Spearman method. The r value is reported for all linear regressions. Multivariable logistic regression was used to rule out covariation between the collected parameters.
For each inflammatory marker, subjects were dichotomized (normal or elevated) according to the upper limits of the core laboratory’s reference ranges of hsCRP >5.0 mg/L, SAA >.97 mg/dL, and fibrinogen >400 mg/dL. Univariate analysis was performed to assess the independent contribution of the risk factors to adverse cardiac and graft-related events.
All variables with P < .10 on univariate analysis were placed into a multivariable Cox proportional hazard model. The Kaplan-Meier method was used to compare the event-free survival rate among subjects, and comparisons were made with the log-rank test. For all event-rate analysis, subjects not experiencing an adverse event were censored at the conclusion of the study (December 31, 2005) as being alive with a patent graft and not experiencing any event. P values were two-tailed, and P < .05 is reported as significant. Statistical analyses were performed on Inter-cooled Stata 7.0 (StataCorp, College Station, Tex).
RESULTS
Patient demographics and risk factors
Subject demographics and risk factor profiles are presented in Table I. Briefly, of the 91 patients enrolled, 63 were men (69.2%); 79 were white (86.8%), 7 were African American (7.7%), and 5 were Hispanic (5.5%); 48 (53%) had diabetes mellitus, 44 (49.4%) had a known history of coronary artery disease, 5 (5.5%) had end-stage renal disease, 74 (81.3%) had hypertension, 73 (80%) were either current or former smokers, and 65 (72%) were currently taking a HMG CoA reductase inhibitor.
Table I.
Characteristics of study population at baseline
| Characteristic | N (%) |
|---|---|
| Age (mean ± SD) | 63 ± 11.5 |
| Male sex | 63 (69) |
| Race | |
| White | 79 (86.8) |
| African American | 7 (7.7) |
| Hispanic | 5 (5.5) |
| CAD | 44 (49.4) |
| Diabetes mellitus | 48 (52.8) |
| ESRD | 5 (5.5) |
| Smoking history | 73 (81.0) |
| Hypertension | 74 (81.3) |
| Current statin use | 65 (72.2) |
| CLI | 50 (55.0) |
CAD, Coronary artery disease; ESRD, end-stage renal disease; CLI, critical limb ischemia.
Surgical procedures
The indication for the revascularization was critical limb ischemia, defined as rest pain or tissue loss, in 50 subjects, claudication in 32, and repair of a popliteal aneurysm in nine. Autogenous conduits included a single segment of great saphenous vein (reversed or nonreversed) in 72, spliced great saphenous or short saphenous vein in 5, single-segment arm vein in 5, and spliced arm vein in 9. Inflow sites included the common femoral artery in 56, superficial femoral artery in 21, or popliteal artery in 14. Distal anastomoses were performed to the popliteal artery in 44, tibial in 27, or pedal vessels in 20. Early (30-day) graft failure occurred in two patients.
Markers of inflammation
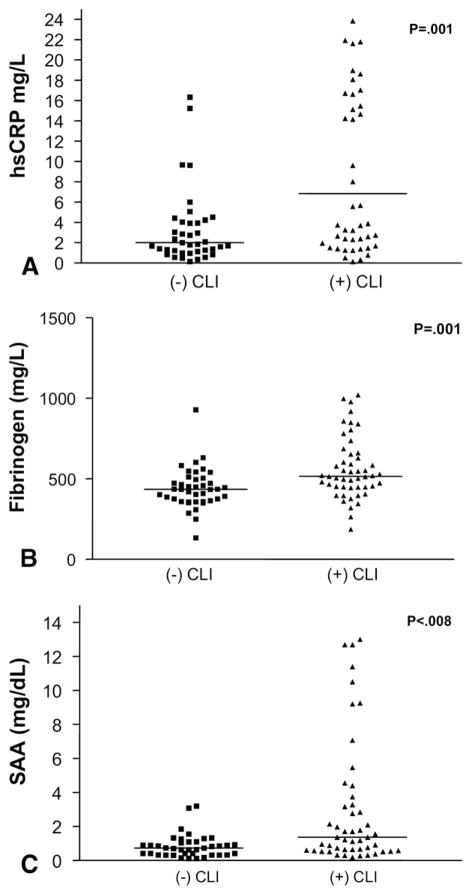
Baseline levels of the inflammatory markers are summarized in Table II. Median hsCRP was 3.2 mg/L (interquartile range, 1.4 to 15), SAA was 0.91 mg/dL (interquartile range, 0.52 to 2.0), and fibrinogen was 472 mg/dL (interquartile range, 396 to 569). Levels of all three inflammatory markers were higher in the patients operated on for critical limb ischemia than for non-critical limb ischemia: hsCRP, 6.84 vs 2.02 mg/L (P = .001); SAA, 1.37 vs 0.74 mg/dL (P = .008); and fibrinogen, 516.2 vs 434.4 mg/dL (P = .001) (Fig 1). High-sensitivity CRP was positively correlated with fibrinogen (r = 0.68, P < .001) and SAA (r = 0.75, P < .001).
Table II.
Inflammatory marker levels in the study population
| N | ABI | CRP (mg/L) | Fibrinogen (mg/dL) | SAA (mg/dL) | |
|---|---|---|---|---|---|
| LEB-all | 91 | 0.51 | 3.2 (1.4–15) | 472 (396–569) | .91 (.52–2.0) |
| LEB/(+) CLI | 41 | 0.51 | 6.84 (2.12–20.3)* | 516.2 (447.6–656.3)* | 1.37 (.59–3.5)* |
| LEB/(−) CLI | 50 | 0.49 | 2.02 (1.19–4.34)* | 434.4 (361.3–500.2)* | .74 (.37–1.1)* |
| LEB/(+) event | 39 | 0.43 | 6.02 (1.6–17.4)* | 465.1 (404.1–639.6) | .9 (.54–4.1) |
| LEB/(−) event | 52 | 0.59 | 2.74 (1.4–5.4)* | 473.6 (394.9–550.3) | .9 (.52–1.5) |
ABI, Ankle-brachial index; CRP, C-reactive protein; SAA, serum amyloid A; LEB, lower-extremity bypass; CLI, critical limb ischemia (rest pain or tissue loss).
All data are shown as median (interquartile range) except ABI (mean).
Values are significant between (+)/(−) subgroups (P < .01).
Fig 1.
Scatter plots indicate preoperative levels of inflammatory markers from 91 patients presenting for lower extremity bypass with critical and noncritical limb ischemia (CLI). A, High-sensitivity C-reactive protein (hsCRP) (mg/L). B, Fibrinogen (mg/dL). C, Serum amyloid A (SAA) (mg/dL). Data are presented as the median and interquartile range.
On Spearman analysis (Table III), diabetes mellitus and critical limb ischemia were significantly correlated with hsCRP, SAA, and fibrinogen. The presence of end-stage renal disease was correlated with hsCRP and SAA but not fibrinogen. Age, smoking, and statin use were not significantly correlated with hsCRP.
Table III.
Spearman correlations between serum amyloid A, high sensitive CRP, and fibrinogen with baseline variables
| Baseline Variable | Spearman r with SAA (p-value) | Spearman r with hsCRP (p-value) | Spearman r with Fib (p-value) |
|---|---|---|---|
| age | −.02 (.84) | .07 (.54) | −.05 (.64) |
| sex | .01 (.89) | −.09 (.41) | −.09 (.39) |
| race | .03 (.78) | .01 (.89) | .14 (.19) |
| CAD | .16 (.14) | .12 (.26) | .02 (.89) |
| DM | .38 (.0002) | .3 (.004) | .27 (.009) |
| ESRD | .27 (.012) | .3 (.004) | .18 (.09) |
| smoker | −.04 (.74) | −.12 (.25) | −.03 (.81) |
| HTN | .04 (.69) | −.09 (.42) | −.12 (.27) |
| hyperlipidemia | .07 (.52) | −.08 (.47) | −.09 (.38) |
| Statin use | .13 (.24) | −.03 (.75) | −.14 (.20) |
| critical limb ischemia | .28 (.008) | .33 (.001) | .35 (.0007) |
SAA, Serum amyloid A; hsCRP, high-sensitivity C-reactive protein; Fib, fibrinogen; CAD, coronary artery disease; DM, diabetes mellitus; ESRD, end-stage renal disease; CLI, critical limb ischemia; HTN, hypertension.
We performed a forward-building stepwise logistic regression to determine the factors that were independently associated with levels of inflammatory markers. This included age, gender, race, diabetes mellitus, statin use, hypertension, smoking, hyperlipidemia, and the presence of critical limb ischemia in the model. Of these factors, only critical limb ischemia (P < .001), diabetes mellitus (P = .003), and hypertension (P = .003) were significantly associated with CRP. When these same variables were used, diabetes mellitus (P = .004), critical limb ischemia (P = .002), and end-stage renal disease (P = .014) were significantly associated with SAA, and diabetes mellitus (P = .006), critical limb ischemia (P < .001), and hypertension, (P = .006) were significantly associated with fibrinogen.
Relationship between inflammatory markers and adverse events
During a mean follow-up of 342 days (range, 36 to 694; median, 320 days), 41 adverse events occurred in this study. Two subjects had more than one event, but for all time-to-event analysis, only the first one was considered. In the perioperative (30-day) period, there were no deaths or major cardiovascular events. During the subsequent follow-up period, most of the events that occurred (33) were either graft-related (25) or contralateral lower extremity events (8). Four graft events were >75% bypass graft stenosis detected on follow-up duplex ultrasound, 11 were graft revisions, and there were 10 bypass graft occlusions or amputations. Four deaths occurred, three of which were cardiovascular-related and one was unknown.
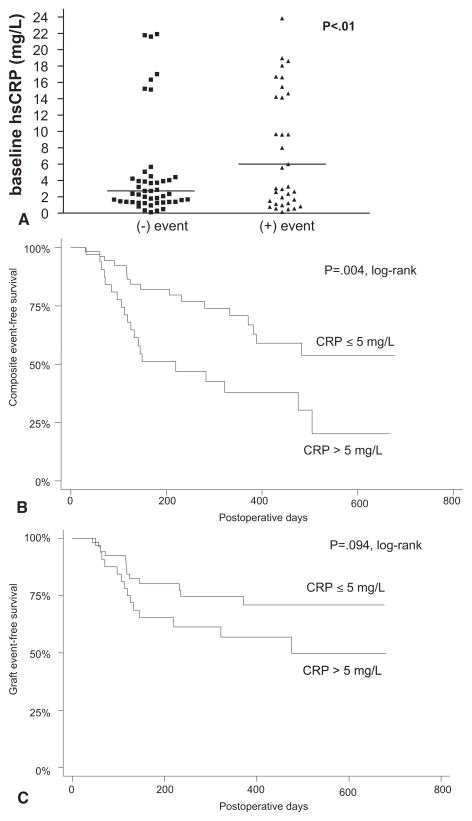
Among patients reaching our final composite end point, 21 (60%) of 35 were in the high-CRP risk category (hsCRP > 5 mg/L), and 18 (32%) of 56 had CRP levels ≤ 5 mg/L (P = .004, log-rank test; Fig 2). Neither SAA (P = .44) nor fibrinogen (P = .49) was associated with adverse events in this cohort of patients. When examining individuals who experienced only graft-related events (without any other cardiovascular end points), 13 (37%) of 35 were in the hsCRP-risk category, and 12 (21%) of 56 were in the lower-risk category (P = .094, log-rank test).
Fig 2.
A, Scatter plot indicates the preoperative level of high-sensitivity C-reactive protein (hsCRP) in 91 patients, and compares those who experienced a postoperative composite end point (myocardial infarction, cerebrovascular accident, death, contralateral limb or graft-related event) with those who did not. Data are presented as the median and interquartile range. B, Freedom from reaching composite end point in subjects with elevated preoperative hsCRP (>5 mg/L) compared with those with levels within the reference range (≤5 mg/L) by Kaplan-Meier method (P = .018; log-rank test). C, Freedom from graft-related events (stenosis, revision, occlusion, or amputation of index leg) in subjects with elevated preoperative hsCRP (>5 mg/L) compared with those with levels within the reference range (≤5 mg/L) by Kaplan-Meier method (P = .094; log-rank test).
A multivariable Cox regression analysis was used to evaluate factors predictive of reaching the composite end point. Included in this model were factors with P < .1 on univariate analysis, which were age, nonwhite race, the presence of coronary artery disease, and critical limb ischemia as the reason for having lower extremity revascularization, and a plasma hsCRP level >5 mg/L. Of these variables, only a high plasma CRP level reached significance (P = .018, hazard ratio, 2.28; 95% confidence interval, 1.15 to 4.51) in the final model. Of note, elevated levels of fibrinogen or SAA were not predictive in this analysis.
DISCUSSION
In this prospective evaluation of patients undergoing lower extremity bypass surgery with autogenous vein, our data show that higher plasma circulating levels of biochemical markers of systemic inflammation—hsCRP, SAA, and fibrinogen—are associated with increased severity of PAD at presentation (ie, critical vs noncritical limb ischemia). Further, in this cohort of patients, baseline hsCRP levels independently predicted a composite end point of major adverse postoperative events, including cardiovascular, contralateral limb, and graft-related end points. By comparison, neither SAA (P = .44) nor fibrinogen (P = .49) was significantly associated with these end points.
To our knowledge, this is the first report of a role for hsCRP in clinical risk prediction among a cohort of patients exclusively undergoing bypass surgery for lower extremity ischemia. These patients, with the possible exception of those presenting with popliteal artery aneurysms, have a substantial burden of atherosclerosis and, arguably, are among the most at-risk group for serious adverse cardiovascular, limb, and graft-related events.
Although agreement is not universal,39 CRP has been predictive of restenosis and cardiovascular events in other similar cohorts undergoing revascularization procedures. For example, hsCRP levels before and after intervention have been associated with 6-month restenosis rates after femoropopliteal angioplasty.40–42 This is consistent with the predictive ability of preangioplasty plasma hsCRP levels for restenosis43 as well as death and myocardial infarction in patients undergoing percutaneous interventions in the coronary circulation.44 In a group of patients undergoing aortocoronary bypass grafting, preoperative elevated levels of hsCRP (CRP ≥3 mg/L) were associated with an increased risk of recurrent ischemia for up to 6 years after the procedure.45
In this study, we used a primary composite end point that included atherosclerotic-related events (myocardial infarction, stroke, death, and contralateral limb events) as well as vein-graft disease, which has been considered more related to myointimal hyperplasia. It is noteworthy that when evaluating only the graft-related end points of stenosis, revision, and occlusion, there was a trend toward lower CRP values predicting freedom from adverse events (P = .094).
We believe that the lack of significance in this subanalysis is due to inadequate power. As enrollment continues to accrue in this study (total planned, N = 300), we will continue to evaluate this relationship. Nevertheless, this should not distract from the significant, independent predictive ability of hsCRP in relation to adverse events in this cohort. We chose an hsCRP value of >5 mg/L as the cutoff for dichotomization of this cohort because it represents the upper limit of normal in our laboratory. Others have divided the hsCRP levels into quartiles or quintiles24,46; however, our small sample size precluded us from determining any meaningful level of significance when subdividing the population further.
Taken together, these data are a compelling implication of inflammation, as measured by plasma levels of hsCRP, in the pathogenesis of restenosis after surgical intervention as well as the development of de novo atherosclerosis-related complications. Chronic inflammation has been implicated in nearly every step in the formation of the atherosclerotic process, as reviewed in detail elsewhere.47,48 However, the potential role of inflammation in the mechanisms of vein graft failure are far more speculative. Acute injury to the vessel wall is followed by an inflammatory response after both percutaneous revascularization49 and saphenous vein bypass grafting.16,50
Whether circulating hsCRP serves as just a marker or plays a direct biologic role in lesion development remains an area of active investigation. Jabs et al51 demonstrated CRP staining by immunohistochemistry in diseased human saphenous vein grafts harvested after aortocoronary bypass surgery. The CRP colocalized with α-actin staining cells of the neointima. These investigators concluded that the CRP expression was due to local production within the vein graft’s wall and that the smooth muscle cell was the likely site of origin.51 However, the origin and biologic relevance of CRP in the vessel wall remains an area of controversy and ongoing study.
There are limitations of this study that merit consideration. With only 91 subjects and <1 year of mean follow-up, our study lacks sufficient power to definitely evaluate markers such as SAA and fibrinogen or to fully assess the ability of hsCRP for accurate risk stratification. Further, dichotomizing hsCRP at 5 mg/L may not be the best discriminatory level for this population of patients. Indeed, the American Heart Association considers individuals with hsCRP values >3 mg/L to be at a high cardiovascular risk.52 The median hsCRP value in our population was 3.2 mg/L, however, thus firmly placing our patients in the high-risk category. Continued re-evaluation as our study accrues and we have longer follow-up times will be needed to refine the predictive abilities of hsCRP.
CONCLUSION
We believe these results have direct clinical relevance. The hsCRP assay may be useful to identify a subgroup within a cohort of PAD patients that are already among the most disadvantaged in terms of cardiovascular morbidity and mortality. This may have implications for the aggressiveness of perioperative medical therapies, behavioral modifications, and, potentially, for bypass graft surveillance.
Indeed, a joint conference sponsored by the Centers for Disease Control and Prevention and the American Heart Association recommended measurement of hsCRP in patients judged to be at intermediate risk by global risk assessment (10% to 20% risk of coronary heart disease per 10 years), level of evidence IIa, class B.53 Although our patients are assumed to have coronary artery disease, occult or overt,1 this may help to identify people in whom more intensive lipid-lowering or antiplatelet regimens may be selected, as well as more aggressive lifestyle modification and better compliance with existing medical therapies.
Further studies will be required to more clearly delineate the relationships between systemic inflammation, conventional risk factors, and the patterns of adaptive or mal-adaptive remodeling within vein bypass grafts.
Acknowledgments
Supported by grant R01 HL 75771 (MAC, MSC), and Clinical Investigator Training Program (C. D. O.).
Footnotes
Competition of interest: Dr Ridker is listed as a co-inventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease.
Presented at The Society for Vascular Surgery, Vascular Annual Meeting, Philadelphia, Pa, June 1 to 4, 2006.
AUTHOR CONTRIBUTIONS
Conception and design: CO, PR, MB, AH, FP, MAC, MSC
Analysis and interpretation: CO, PR, MB, AH, FP, MAC, MSC, FL
Data collection: CO, PR, MSC, MAC
Writing the article: CO, PR, MSC, MAC
Critical revision of the article: CO, PR, MB, AH, FP, MAC, MSC, FL
Final approval of the article: CO, PR, MB, AH, FP, MAC, MSC, FL
Statistical analysis: CO, MSC, MAC
Obtained funding: CO, PR, MSC, MAC
Overall responsibility: CO
References
- 1.Criqui MH. Peripheral arterial disease—epidemiological aspects. Vasc Med. 2001;6:3–7. doi: 10.1177/1358836X0100600i102. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, Mc-Cann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 6.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 7.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837–42. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 8.Koenig W, Khuseyinova N, Lowel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation. 2004;110:1903–8. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 9.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 10.Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation. 2003;108:2292–7. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- 12.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 14.Taylor LM, Jr, Edwards JM, Porter JM. Present status of reversed vein bypass grafting: five-year results of a modern series. J Vasc Surg. 1990;11:193–205. doi: 10.1067/mva.1990.17235. discussion 205-196. [DOI] [PubMed] [Google Scholar]
- 15.Conte MS, Belkin M, Upchurch GR, Mannick JA, Whittemore AD, Donaldson MC. Impact of increasing comorbidity on infrainguinal reconstruction: a 20-year perspective. Ann Surg. 2001;233:445–52. doi: 10.1097/00000658-200103000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox JL, Chiasson DA, Gotlieb AI. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34:45–68. doi: 10.1016/0033-0620(91)90019-i. [DOI] [PubMed] [Google Scholar]
- 17.Hoch JR, Stark VK, Hullett DA, Turnipseed WD. Vein graft intimal hyperplasia: leukocytes and cytokine gene expression. Surgery. 1994;116:463–70. discussion 470-61. [PubMed] [Google Scholar]
- 18.Stark VK, Hoch JR, Warner TF, Hullett DA. Monocyte chemotactic protein-1 expression is associated with the development of vein graft intimal hyperplasia. Arterioscler Thromb Vasc Biol. 1997;17:1614–21. doi: 10.1161/01.atv.17.8.1614. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Peppel K, Brian L, Chien L, Freedman NJ. Vein graft neointimal hyperplasia is exacerbated by tumor necrosis factor receptor-1 signaling in graft-intrinsic cells. Arterioscler Thromb Vasc Biol. 2004;24:2277–83. doi: 10.1161/01.ATV.0000147766.68987.0d. [DOI] [PubMed] [Google Scholar]
- 20.Abbruzzese TA, Havens J, Belkin M, Donaldson MC, Whittemore AD, Liao JK, et al. Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts. J Vasc Surg. 2004;39:1178–85. doi: 10.1016/j.jvs.2003.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 22.Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–42. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–8. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–5. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 26.Wildman RP, Muntner P, Chen J, Sutton-Tyrrell K, He J. Relation of inflammation to peripheral arterial disease in the national health and nutrition examination survey, 1999–2002. Am J Cardiol. 2005;96:1579–83. doi: 10.1016/j.amjcard.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 27.Beckman JA, Preis O, Ridker PM, Gerhard-Herman M. Comparison of usefulness of inflammatory markers in patients with versus without peripheral arterial disease in predicting adverse cardiovascular outcomes (myocardial infarction, stroke, and death) Am J Cardiol. 2005;96:1374–8. doi: 10.1016/j.amjcard.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 28.McDermott MM, Guralnik JM, Corsi A, Albay M, Macchi C, Bandinelli S, et al. Patterns of inflammation associated with peripheral arterial disease: the InCHIANTI study. Am Heart J. 2005;150:276–81. doi: 10.1016/j.ahj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 29.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005;112:976–83. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- 30.McDermott MM, Greenland P, Green D, Guralnik JM, Criqui MH, Liu K, et al. D-dimer, inflammatory markers, and lower extremity functioning in patients with and without peripheral arterial disease. Circulation. 2003;107:3191–8. doi: 10.1161/01.CIR.0000074227.53616.CC. [DOI] [PubMed] [Google Scholar]
- 31.McDermott MM, Guralnik JM, Greenland P, Green D, Liu K, Ridker PM, et al. Inflammatory and thrombotic blood markers and walking-related disability in men and women with and without peripheral arterial disease. J Am Geriatr Soc. 2004;52:1888–94. doi: 10.1111/j.1532-5415.2004.52514.x. [DOI] [PubMed] [Google Scholar]
- 32.Vainas T, Stassen FR, de Graaf R, Twiss EL, Herngreen SB, Welten RJ, et al. C-reactive protein in peripheral arterial disease: relation to severity of the disease and to future cardiovascular events. J Vasc Surg. 2005;42:243–51. doi: 10.1016/j.jvs.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 33.Vainas T, Lubbers T, Stassen FR, Herngreen SB, van Dieijen-Visser MP, Bruggeman CA, et al. Serum C-reactive protein level is associated with abdominal aortic aneurysm size and may be produced by aneurysmal tissue. Circulation. 2003;107:1103–5. doi: 10.1161/01.cir.0000059938.95404.92. [DOI] [PubMed] [Google Scholar]
- 34.Rossi E, Biasucci LM, Citterio F, Pelliccioni S, Monaco C, Ginnetti F, et al. Risk of myocardial infarction and angina in patients with severe peripheral vascular disease: predictive role of C-reactive protein. Circulation. 2002;105:800–3. doi: 10.1161/hc0702.104126. [DOI] [PubMed] [Google Scholar]
- 35.Doweik L, Maca T, Schillinger M, Budinsky A, Sabeti S, Minar E. Fibrinogen predicts mortality in high risk patients with peripheral artery disease. Eur J Vasc Endovasc Surg. 2003;26:381–6. doi: 10.1016/s1078-5884(03)00340-x. [DOI] [PubMed] [Google Scholar]
- 36.Wattanakit K, Folsom AR, Selvin E, Weatherley BD, Pankow JS, Brancati FL, et al. Risk factors for peripheral arterial disease incidence in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2005;180:389–97. doi: 10.1016/j.atherosclerosis.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 37.Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:726–32. doi: 10.1161/01.CIR.0000115516.54550.B1. [DOI] [PubMed] [Google Scholar]
- 38.Rifai N, Joubran R, Yu H, Asmi M, Jouma M. Inflammatory markers in men with angiographically documented coronary heart disease. Clin Chem. 1999;45:1967–73. [PubMed] [Google Scholar]
- 39.Rittersma SZ, de Winter RJ, Koch KT, Schotborgh CE, Bax M, Heyde GS, et al. Preprocedural C-reactive protein is not associated with angiographic restenosis or target lesion revascularization after coronary artery stent placement. Clin Chem. 2004;50:1589–96. doi: 10.1373/clinchem.2004.032656. [DOI] [PubMed] [Google Scholar]
- 40.Schillinger M, Exner M, Mlekusch W, Rumpold H, Ahmadi R, Sabeti S, et al. Vascular inflammation and percutaneous transluminal angioplasty of the femoropopliteal artery: association with restenosis. Radiology. 2002;225:21–6. doi: 10.1148/radiol.2251011809. [DOI] [PubMed] [Google Scholar]
- 41.Tschopl M, Tsakiris DA, Marbet GA, Labs KH, Jager K. Role of hemostatic risk factors for restenosis in peripheral arterial occlusive disease after transluminal angioplasty. Arterioscler Thromb Vasc Biol. 1997;17:3208–14. doi: 10.1161/01.atv.17.11.3208. [DOI] [PubMed] [Google Scholar]
- 42.Schillinger M, Haumer M, Schlerka G, Mlekusch W, Exner M, Ahmadi R, et al. Restenosis after percutaneous transluminal angioplasty in the femoropopliteal segment: the role of inflammation. J Endovasc Ther. 2001;8:477–83. doi: 10.1177/152660280100800509. [DOI] [PubMed] [Google Scholar]
- 43.Buffon A, Liuzzo G, Biasucci LM, Pasqualetti P, Ramazzotti V, Rebuzzi AG, et al. Preprocedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J Am Coll Cardiol. 1999;34:1512–21. doi: 10.1016/s0735-1097(99)00348-4. [DOI] [PubMed] [Google Scholar]
- 44.Chew DP, Bhatt DL, Robbins MA, Penn MS, Schneider JP, Lauer MS, et al. Incremental prognostic value of elevated baseline C-reactive protein among established markers of risk in percutaneous coronary intervention. Circulation. 2001;104:992–7. doi: 10.1161/hc3401.095074. [DOI] [PubMed] [Google Scholar]
- 45.Milazzo D, Biasucci LM, Luciani N, Martinelli L, Canosa C, Schiavello R, et al. Elevated levels of C-reactive protein before coronary artery bypass grafting predict recurrence of ischemic events. Am J Cardiol. 1999;84:459–61. A9. doi: 10.1016/s0002-9149(99)00333-1. [DOI] [PubMed] [Google Scholar]
- 46.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–33. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 47.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 48.Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–71. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 49.Schillinger M, Exner M, Mlekusch W, Haumer M, Ahmadi R, Rumpold H, et al. Balloon angioplasty and stent implantation induce a vascular inflammatory reaction. J Endovasc Ther. 2002;9:59–66. doi: 10.1177/152660280200900111. [DOI] [PubMed] [Google Scholar]
- 50.Eslami MH, Gangadharan SP, Belkin M, Donaldson MC, Whittemore AD, Conte MS. Monocyte adhesion to human vein grafts: a marker for occult intraoperative injury? J Vasc Surg. 2001;34:923–9. doi: 10.1067/mva.2001.118590. [DOI] [PubMed] [Google Scholar]
- 51.Jabs WJ, Theissing E, Nitschke M, Bechtel JF, Duchrow M, Mohamed S, et al. Local generation of C-reactive protein in diseased coronary artery venous bypass grafts and normal vascular tissue. Circulation. 2003;108:1428–31. doi: 10.1161/01.CIR.0000092184.43176.91. [DOI] [PubMed] [Google Scholar]
- 52.Pearson TA, Mensah GA, Hong Y, Smith SC., Jr CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: application to clinical and public health practice: overview. Circulation. 2004;110:e543–4. doi: 10.1161/01.CIR.0000148979.11121.6B. [DOI] [PubMed] [Google Scholar]
- 53.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]