Abstract
IL-1 has been associated with acute lung injury (ALI) in both humans and animal models, but further investigation of the precise mechanisms involved is needed, and may identify novel therapeutic targets. To discover the IL-1 mediators essential to the initiation and resolution phases of acute lung inflammation, knockout mice (with targeted deletions for either the IL-1 receptor–1, i.e., Il-1r1−/−, or the IL-1 receptor antagonist, i.e., Il-1rn−/−) were exposed to aerosolized LPS, and indices of lung and systemic inflammation were examined over the subsequent 48 hours. The resultant cell counts, histology, protein, and RNA expression of key cytokines were measured. Il-1r1−/− mice exhibited decreased neutrophil influx, particularly at 4 and 48 hours after exposure to LPS, as well as reduced bronchoalveolar lavage (BAL) expression of chemokines and granulocyte colony–stimulating factor (G-CSF). On the contrary, Il-1rn−/− mice demonstrated increased BAL neutrophil counts, increased BAL total protein, and greater evidence of histologic injury, all most notably 2 days after LPS exposure. Il-1rn−/− mice also exhibited higher peripheral neutrophil counts and greater numbers of granulocyte receptor-1 cells in their bone marrow, potentially reflecting their elevated plasma G-CSF concentrations. Furthermore, IL-17A expression was increased in the BAL and lungs of Il-1rn−/− mice after exposure to LPS, likely because of increased numbers of γδ T cells in the Il-1rn−/− lungs. Blockade with IL-17A monoclonal antibody before LPS exposure decreased the resultant BAL neutrophil counts and lung G-CSF expression in Il-1rn−/− mice, 48 hours after exposure to LPS. In conclusion, Il-1rn−/− mice exhibit delayed resolution in acute lung inflammation after exposure to LPS, a process that appears to be mediated via the G-CSF/IL-17A axis.
Keywords: IL-1 receptor antagonist, IL-17A, IL-1 receptor–1, acute lung injury
Clinical Relevance
The precise mechanisms by which IL-1 is involved in the development of acute lung injury remain poorly understood. Moreover, the discovery of IL-1 pathway mediators essential for lung inflammation may offer new therapeutic targets. We found that whereas Il-1r1−/− mice (with targeted deletions for IL-1 receptor–1) exhibited less lung inflammation after exposure to LPS, Il-1rn−/− mice (with targeted deletions for the IL-1 receptor antagonist) demonstrated delayed resolution of injury, which may be mediated via the expression of IL-17A/granulocyte colony–stimulating factor.
IL-1 is a potent proinflammatory molecule, and genetic polymorphisms in the IL-1 pathway have been associated with a diverse group of conditions, including several lung diseases (1–4). Recent data suggest an alteration in susceptibility to developing acute lung injury (ALI), characterized by bilateral pulmonary infiltrates with significantly impaired oxygenation, in several cohorts of patients possessing a polymorphism in the IL-1RN gene (which encodes the IL-1 receptor antagonist protein, IL-1RA) (5). Although the specific functional ramifications of these polymorphisms remain unclear, other human studies have also suggested a link between ALI and the IL-1 pathway. One such study reported that IL-1β protein concentrations were higher in the bronchoalveolar lavage (BAL) of subjects who developed adult respiratory distress syndrome (ARDS) than in control subjects, and those subjects with ARDS with low concentrations of IL-1RA were more likely to die (6). More is known, however, regarding the role of IL-1 pathway components in other inflammatory diseases. Notably, a recombinant human (rh) form of IL-1RA (commercially available as anakinra) is approved for clinical use in the treatment of adults with moderate to severe rheumatoid arthritis (7). Furthermore, although a trial of rhIL-1RA infusion in sepsis did not result in an overall decrease in mortality, that trial reported provocative results, in which mortality was significantly reduced in several subgroups that received rhIL-1RA, including subjects with ARDS (8).
The key steps in activating the IL-1 cascade begin with the binding of the agonists IL-1α or IL-1β to IL-1 receptor–I (which encodes the IL-1R1 protein; the Il-1R1 gene). Although both IL-1α and IL-1β activate IL-1R1, the expression patterns of these agonists vary, and their relative contributions to pathogenesis differ among cell types (9, 10). The binding of either IL-1 agonist allows the recruitment of the IL-1 receptor accessory protein, followed by additional adaptor proteins. Subsequently, signal transduction occurs via the NF-κB, mitogen-activated protein kinase, c-Jun N-terminal kinase, and p38 pathways. The downstream consequences of IL-1 pathway activation include the up-regulation of a cascade of inflammatory mediators. This includes the increased expression of cytokines and chemokines that regulate many aspects of the immune response, particularly CXC1, CXCL2, IL-6, granulocyte colony–stimulating factor (G-CSF), and IL-17A (10, 11). However, which of these specific mediators play a critical role in the cascade initiated by IL-1 in ALI in humans remains unclear.
A particularly promising family of proinflammatory cytokines induced by IL-1 (which we hypothesize to be involved in the pathogenesis of ALI) comprises the IL-17 family, previously implicated in several immune-mediated diseases. IL-17A (encoded by the Il-17a gene) is the most well studied member of this pathway, and binds a widely expressed IL-17RA-RC receptor (12). In the past few years, IL-17A was found to be produced not only by Th17 cells, but also by innate immune cells, including γδ T cells (13, 14). IL-17A can act indirectly on neutrophils, cells that have been demonstrated as crucial for host defense and for the development of ALI (13, 15, 16). IL-17A influences neutrophil function by up-regulating CXCL1 and G-CSF, both of which help recruit neutrophils to the site of injury. IL-17F is a structurally related ligand that binds with less affinity to the same IL-17RA-RC receptor, and has a similar functional profile, playing a role in host defense against pathogens as well as in autoimmune diseases (13, 17). Although most investigations of IL-17 family members have focused on rheumatologic disease, IL-17A and IL-17F were found to influence disease in several pulmonary conditions, although primarily in murine models (18–20). Specifically, the up-regulation of IL-17A and IL-17F has been demonstrated in mice with pneumonia attributable to Pseudomonas aeruginosa, Klebseilla pneumoniae, and H1N1 influenza virus (21–23).
In addition to IL-1 inducing the expression of several proinflammatory mediators, IL-1 is also thought to stimulate the production of counterregulatory molecules including IL-1RA, the inhibitor of its own pathway. IL-1RA competes for binding to IL-1R1 with the IL-1 agonists. The IL-1RA protein exists in three isoforms (produced by alternative splicing) in humans and mice, that is, one secreted (sIL-1RA) and two intracellular (icIL-1RA) forms. sIL-1RA is released extracellularly during inflammatory events, whereas icIL-1RA exerts transcriptional control in the nucleus, but may serve a role similar to that of sIL-1RA when released from dying cells (9, 10). Human and murine forms of the sIL-1RA protein share 77% homology (24).
To determine the mechanisms by which the IL-1 pathway might influence the development of ALI, we examined the inflammatory response to LPS in mice deficient in Il-1r1 (i.e., unable to activate the IL-1 pathway) and mice with a targeted deletion in the Il-1rn gene that do not produce a secreted form of the IL-1 receptor antagonist protein. We hypothesized that alterations in the extent and timing of lung inflammation in the loss of function Il-1r1−/− mice and gain of function Il-1rn−/− mice would yield important clues in regard to how the IL-1 pathway contributes to ALI. Some of the results of this study were previously reported in the form of abstracts at the American Thoracic Society (5, 25, 26).
Materials and Methods
Detailed methods are available in the Methods section of the online supplement.
Animals
C57BL/6 (wild-type controls) and Il-1r1−/− and Il-1rn−/− mice (on a C57BL/6 background) in breeding pairs were purchased from Jackson Laboratories (Bar Harbor, ME). All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Children's Hospital of Philadelphia.
LPS Exposure and In Vivo Sample Collection
Mice were exposed to aerosolized LPS, at a dose of approximately 3 μg/mouse (27). Blood, BAL, and lung tissue were harvested as previously detailed (28). A subgroup of mice was pretreated with rhIL-1RA (anakinra, i.e., Kineret; Biovitrum, Stockholm, Sweden) or PBS control intratracheally, 2 hours before LPS exposure. Another subgroup was pretreated with IL-17A monoclonal antibody (mAb) (R&D Systems, Minneapolis, MN) or isotype control antibody (Ab) intraperitoneally, 24 hours before exposure to LPS (29).
Flow Cytometry
Bone marrow cells and lung cells were collected, stained, and analyzed as described in the online supplement. Notably, > 95% of cells were viable in all experiments.
Cytokine Measurement
Cytokine protein concentration was measured in BAL or plasma by ELISA. Real-time PCR provided a relative quantification of mRNA cytokine expression in the lung, as described in the Methods section of the online supplement.
Statistical Analysis
Cell counts, protein, and RNA expression were compared overall using two-way ANOVA (represented by P values above graphs), and with the Bonferroni post hoc test to assess the significance of individual time points (P < 0.05, denoted by an asterisk within graphs). Note that in Figures 2E, 4A, E3B, E7A, and E7C, the results may not have been significant overall, according to two-way ANOVA, but individual time points were significant according to the Bonferroni post hoc test. The remaining data were analyzed according to unpaired Student t test, as outlined in the online supplement. All data expressed as means ± SEM.
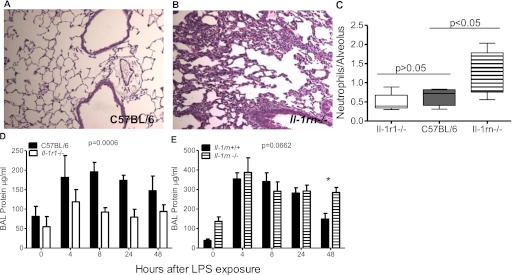
Figure 2.
BAL total protein and lung histology after exposure to LPS. Hematoxylin and eosin–stained sections of lungs fixed 48 hours after LPS exposure at ×10 show (A) minimal inflammatory cells present in the alveolar space or interstitium of C57BL/6 murine lungs, but (B) substantial cellular infiltrates in Il-1rn−/− murine lungs. (C) Semiquantitative analysis of the number of neutrophils counted per alveolus in Il-1rn−/− mice 48 hours after exposure to LPS, compared with C57BL/6 mice (five mice per genotype, 10 fields counted per mouse). (D) BAL total protein in Il-1r1−/− mice (n = 10–12 mice per time point). (E) BAL total protein in Il-1rn−/− mice was increased exclusively at 48 hours (P < 0.05) after exposure to LPS (n = 9–16 mice per time point).
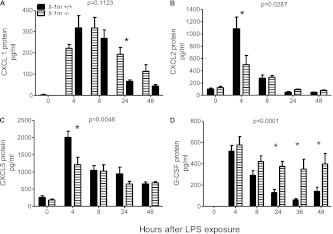
Figure 4.
BAL cytokine expression in Il-1rn−/− mice after exposure to LPS. (A) CXCL1 was exclusively elevated at 24 hours (*P < 0.05) in Il-1rn−/− mice, as opposed to littermate control mice (Il-1rn+/+). (B) CXCL2 at 4 hours (*P < 0.001). (C) CXCL5 at 4 hours (*P < 0.001). (D) G-CSF expression at 24 hours (*P < 0.05), 36 hours (*P < 0.05), and 48 hours (*P < 0.01) after exposure to LPS (n = 10–19 mice per time point).
Results
Increased Expression of IL-1β and IL-1RA in LPS-Induced Acute Lung Inflammation
The potential involvement of the IL-1 pathway in LPS-induced lung inflammation was examined by assessing the temporal response of IL-1β, IL-1α, and IL-1RA in the BAL and lung tissue of C57BL/6 mice. Both BAL protein and lung mRNA concentrations of IL-1β and IL-1RA increased after aerosolized LPS exposure (Figures E1A and E1B in the online supplement). Although IL-1RA protein expression in BAL paralleled its mRNA time course, the IL-1β protein increase in BAL was delayed compared with Il-1β mRNA in the lung (Figure E1), likely reflecting additional controls on the release of IL-1β via the inflammasome (10, 30). The observed increase in Il-1α mRNA soon after exposure to LPS, without an appreciable elevation in IL-1α protein in the BAL, is consistent with a predominately intracellular role for IL-1α (9).
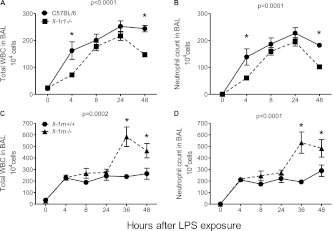
BAL Neutrophils Decreased in Il-1r1−/− Mice and Increased in Il-1rn−/− Mice after Exposure to LPS
In response to inhaled LPS, as we and others previously reported, neutrophils are recruited into the lungs, and acute lung inflammation ensues in a self-limited manner (27, 31, 32). To determine the contribution of the IL-1 pathway to inflammatory cell accumulation, we compared bronchoalveolar cell counts in wild-type mice to those deficient in IL-1R1. Figures 1A and 1B illustrate that the total leukocyte (WBC) and neutrophil counts in BAL were decreased at all time points after exposure to LPS in Il-1r1−/− mice compared with C57BL/6 control mice. This difference was most pronounced at 4 and 48 hours after LPS inhalation. The delayed accumulation of WBCs, particularly neutrophils, in Il-r1−/− mice suggests that the activation of this receptor may be important early in the course of pulmonary neutrophil influx. Moreover, the more rapid resolution of neutrophil numbers also implies a role for IL-1R1 during this phase.
Figure 1.
Bronchoalveolar lavage (BAL) cell counts after LPS exposure. (A) Overall (analyzed by two-way ANOVA, significance indicated by P value at top of graph) total leukocytes (WBC), at 4 hours (analyzed by Bonferroni post hoc test, *P < 0.01) and at 48 hours (*P < 0.001), as well as (B) neutrophil counts overall in Il-1r1−/− mice (with targeted deletions for IL-1 receptor–1), at 4 hours (*P < 0.05) and at 48 hours (*P < 0.01), compared with C57BL/6 control mice after exposure to LPS (n = 10–12 mice per time point). (C) Total WBC at 36 hours (*P < 0.0001) as well as 48 hours (*P < 0.01) and (D) total neutrophil counts in Il-1rn−/− mice (with targeted deletions for the IL-1 receptor antagonist), at 36 hours (*P < 0.001) and at 48 hours (*P < 0.01), compared with littermate controls (Il-1rn+/+ mice) after LPS exposure (n = 9–19 mice per time point).
In contrast to Il-1r1−/− mice, mice with a targeted deletion for the secreted form of the IL-1 receptor antagonist protein (Il-1rn−/−) demonstrated significantly greater overall BAL total WBC and neutrophil counts, most notably at 36 and at 48 hours after exposure to LPS (Figures 1C and 1D). These data suggest that without expression of the natural antagonist, the increased IL-1β expression observed at 24–48 hours (Figure E1A) remains unopposed, prolonging the inflammatory cascade and delaying the resolution of injury.
Histology and BAL Total Protein Mirror BAL Cell Count Trends in Il-1r1−/− and Il-1rn−/− Mice
To determine whether evidence of persistent BAL neutrophilia is accompanied by histologic evidence of prolonged lung inflammation in Il-1rn−/− mice, we compared histologic sections of fixed murine lungs, 48 hours after LPS exposure. As seen in Figure 2A, an almost complete resolution of alveolar or interstitial cellular infiltrates was evident in C57BL/6 lungs. Likewise, cross sections of Il-1r1−/− lungs (not shown) were indistinguishable from lungs of C57BL/6 mice at 48 hours. In contrast, multiple patches of neutrophil-predominant intra-alveolar infiltrates were present in the lungs of Il-1rn−/− mice, 48 hours after exposure to LPS (Figure 2B). A semiquantitative analysis of neutrophil numbers (determined by counting neutrophils per alveolus) revealed that neutrophils were significantly increased in the lung parenchyma of Il-1rn−/− mice (Figure 2C).
The BAL total protein concentration (a gross measurement of capillary permeability) was also found to be significantly lower in I1–1r1−/− mice than in wild-type control mice (Figure 2D). In contrast, we discovered a significant increase in BAL protein concentration exclusively at 48 hours after exposure to LPS in Il-1rn−/− mice (Figure 2E). Together, these data suggest a delayed resolution of injury in mice lacking the secreted form of the IL-1RA protein.
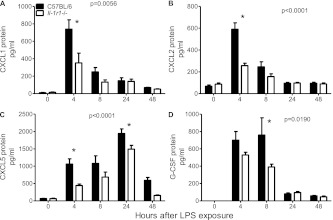
Decreased Expression of BAL CXCL1 and CXCL2 in Il-1r1−/− Mice Early after Exposure to LPS
To determine whether proinflammatory cytokine concentrations in BAL would be altered by the loss of the IL-1 pathway, we measured their protein concentration via ELISA. As seen in Figures 3A and 3B, Il-1r1−/− mice exhibited a significantly reduced protein expression of the neutrophil chemoattractants CXCL1 (keratinocyte-derived chemokine) and CXCL2 (macrophage inflammatory protein-2) overall, with the most pronounced difference in expression levels between the two genotypes measured at 4 hours. Moreover, the expression of CXCL5 (lipopolysaccharide-induced CXC chemokine; a neutrophil chemoattractant we previously showed to be critical in this LPS model of lung inflammation) (31) was also decreased, particularly at 4 hours in Il-1r1−/− mice (Figure 3C). The decreased early expression of CXCL1, CXCL2, and CXCL5 may contribute to the lower numbers of BAL neutrophils observed in Il-1r1−/− mice soon after exposure to LPS. Similarly, the reduced protein concentrations of IL-6 and TNF-α at 4 hours may influence the early inflammatory response in Il-1r1−/− murine lungs (Figures E2A and E2B).
Figure 3.
Cytokine concentration in the BAL of Il-1r1−/− mice after exposure to LPS, compared with C57BL/6 control mice. (A) CXCL1 at 4 hours (*P < 0.0001). (B) CXCL2 at 4 hours (*P < 0.0001). (C) CXCL5 at 4 hours (*P < 0.01) and 48 hours (*P < 0.05). (D) Granulocyte colony–stimulating factor (G-CSF) expression at 8 hours (*P < 0.01; n = 10–12 mice per time point).
Reduced BAL G-CSF and CXCL5 May Contribute to Decreased Neutrophils in Il-1r1−/− Mice 48 Hours after Exposure to LPS
The more rapid resolution of neutrophilic inflammation at 48 hours in the BAL of Il-1r1−/− mice (Figure 1B) suggests the possibility that the IL-1 pathway contributes to this later phase of inflammation, potentially related to the decreased BAL CXCL5 expression at 24 hours (Figure 3C). A lower observed BAL G-CSF protein concentration, particularly at 8 hours (Figure 3D), may also alter neutrophil numbers at later time points, particularly if this BAL G-CSF reflects plasma G-CSF expression, which may influence granulopoiesis (33). Because neutrophils are a significant source of IL-1RA protein, we speculate that the decreased BAL IL-1RA, observed most significantly at 48 hours in Il-1r1−/− mice (Figure E2C), reflects the lower number of neutrophils in the alveolar space at that time (19).
Higher BAL CXCL1 and G-CSF Concentrations May Contribute to Increased Neutrophils in Il-1rn−/− Mice
As opposed to the decreased cytokines seen in Il-1r1−/− mice, we anticipated that the deletion of the key pathway antagonist would result in a heightened cytokine expression of known downstream products, particularly CXCL1 and IL-6 (11). Figure 4A illustrates that a significant elevation of CXCL1 protein occurred in the BAL 24 hours after exposure to LPS, whereas G-CSF was increased at 24, 36, and 48 hours (Figure 4D). These data are consistent with the concept that IL-1RA is a known inhibitor of G-CSF expression (34). We hypothesize that the lack of sIL-1RA in Il-1rn−/− mice results in elevated concentrations of G-CSF and CXCL1 that contribute to the increased alveolar neutrophils seen at 36 and 48 hours after exposure to LPS in Il-1rn−/− mice (Figures 1C and 1D).
Surprisingly, significantly decreased concentrations of the neutrophil chemokines CXCL2 and CXCL5 were evident, and were especially prominent at 4 hours (Figures 4C and 4D). A decrease in TNF-α concentration was also evident in Il-1rn−/− mice, exclusively 4 hours after exposure to LPS (Figure E3B). The decreased expression of these cytokines may be related to chronic inflammation in Il-1rn−/− mice, but warrants further investigation. Contrary to our initial hypothesis, we observed no significant difference in BAL IL-6 expression (Figure E3A). Finally, as anticipated, we confirmed that negligible IL-1RA protein was present in the BAL of Il-1rn−/− mice (Figure E3C).
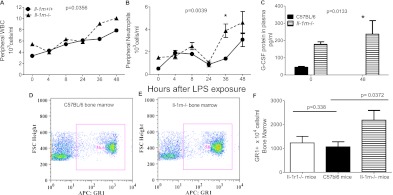
Higher Peripheral Circulating WBCs and Plasma G-CSF in Il-1rn−/− Mice
The increased G-CSF expression in the BAL of Il-1rn−/− mice motivated us to examine the peripheral cell counts for these mice. Figures 5A and 5B depict the significantly higher overall peripheral total WBCs and neutrophils in Il-1rn−/− mice, with the neutrophils significantly higher at 36 hours. To determine whether neutrophilia may reflect the systemic expression of G-CSF, we measured the plasma protein concentration of G-CSF and found a significantly elevated G-CSF concentration in Il-1rn−/− mice overall, compared with C57BL/6 control mice. Moreover, whereas minimal G-CSF was apparent in wild-type mice at 48 hours, Il-1rn−/− mice continued to demonstrate substantially increased concentrations of plasma G-CSF at this time (Figure 5C).
Figure 5.
Differences in peripheral and bone marrow cell counts in Il-1rn−/− mice after exposure to LPS. (A) Total peripheral WBC and (B) peripheral neutrophil counts in Il-1rn−/− mice, compared with littermate control mice (Il-1rn+/+). Peripheral neutrophil counts at 36 hours (*P < 0.05) in Il-1rn−/− mice (n = 9–17 mice per time point). (C) G-CSF protein expression in Il-1rn−/− mice 48 hours (*P < 0.05) after exposure to LPS, compared with C57BL/6 mice (n = 5–7 mice at each time point). (D) Representative dot plots illustrate the relative percentage of GR1+ cells in the unstimulated marrow of C57BL/6 mice, compared with (E) marrow in Il-1rn−/− mice. APC, allophycocyanin; FSC, forward scatter. (F) Absolute number of GR1+ cells in Il-1rn−/− mice versus C57BL/6 mice, as well as GR1+ cells in the bone marrow of Il-1r1−/− versus C57BL/6 mice (n = 3–4 mice per genotype).
In comparison, no difference was evident in peripheral WBCs between Il-1r1−/− mice compared with C57BL/6 control mice (data not shown). Likewise, the peripheral neutrophil counts did not differ between Il-1r1−/− and C57BL/6 mice at baseline or at any time point after exposure to LPS.
Increased Cell Counts May Be Related to Altered Bone Marrow Composition in the Il-1rn−/−Mice
To dissect the etiology further of the higher inflammatory cell counts observed in Il-1rn−/− mice, we examined the cellular composition of their unstimulated bone marrow according to flow cytometry. Figures 5D and 5E are representative dot plots illustrating the striking difference in bone marrow composition of granulocyte receptor-1 (GR1+) cells (a marker of neutrophil lineage) between Il-1rn−/− and C57BL/6 mice at baseline. In addition to the increased percentage of GR1+ cells (Figure E4A), a higher absolute GR1+ cell count was found in Il-1rn−/− mice (Figure 5F), even though these mice had uniformly lower body masses (35). We speculate that the increased number of neutrophils and neutrophil progenitors in Il-1rn−/− bone marrow at baseline has the potential to render these animals more sensitive to chemokine-induced neutrophil recruitment, as incited by injurious stimuli. Although a greater percentage of macrophage lineage cells (F4–80+CD11b+ cells) was observed in the bone marrow of Il-1rn−/− mice (Figure E4B), no difference in absolute macrophage counts (Figure E4C) was evident.
Unlike the Il-1rn−/− mice, no alteration was evident in the absolute count or percentage of GR1+ cells in the baseline bone marrow of Il-1r1−/− mice, compared with the bone marrow of C57BL/6 mice (Figures 5F and E4A). Similarly, no difference was evident in the number or percentage of F4–80+CD11b+ cells in Il-1r1−/− mice compared with C57BL/6 mice (Figures E4B and E4C).
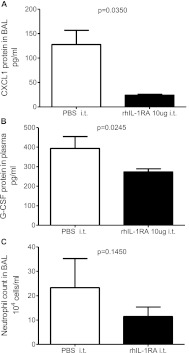
Pretreatment with Intratracheal rhIL-1RA Decreases G-CSF 48 Hours after LPS Exposure
In an attempt to reverse the phenotype of lung inflammation observed in Il-1rn−/− mice, we repleted their alveolar space with rhIL-1RA or PBS. Intratracheal pretreatment with rhIL-1RA resulted in less BAL CXCL1 and plasma G-CSF protein expression 48 hours after exposure to LPS (Figures 6A and 6B). Moreover, a trend toward decreased BAL neutrophil numbers was evident (Figure 6C), although it was not statistically significant. No alterations in BAL total WBC or peripheral cell counts were evident in rhIL-1RA–pretreated mice, nor was a difference in bone marrow composition observed (data no shown). These data further suggest, as observed in Figures 4A and 4D as well as Figure 5C, that a deficiency of sIL-1RA protein contributes to elevated G-CSF and CXCL1 concentrations after LPS exposure in Il-1rn−/− mice.
Figure 6.
Cytokine and cell count response in Il-1rn−/− mice pretreated with recombinant human (rh) IL-1RA before exposure to LPS. Forty-eight hours after exposure to LPS, (A) the BAL expression of CXCL1 and (B) plasma concentration of G-CSF in Il-1rn−/− mice that had been pretreated 2 hours before LPS exposure with 10 μg rhIL-1RA intratracheally were compared with those in mice pretreated with PBS intratracheally (C) Neutrophil numbers in BAL after pretreatment with rhIL-1RA (n = 3–5 mice per treatment).
C57BL/6 mice pretreated with intratracheal rhIL-1RA before exposure to LPS, in the same manner as already described, did not significantly alter BAL neutrophils or BAL WBCs 48 hours after exposure to LPS. Similarly, no significant reduction was observed in G-csf or Il-17a mRNA expression in the lungs of mice pretreated with rhIL-1RA, compared with mice pretreated with PBS (data not shown).
IL-17A Plays a Key Role in Neutrophil Accumulation in Il-1rn−/− Mice
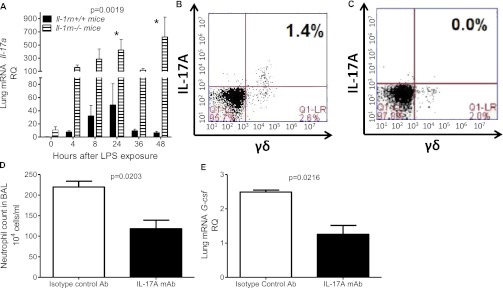
To explore the regulation further of G-CSF in the lungs of Il-1rn−/− mice, we examined the expression of IL-17A, a potent inducer of G-CSF. Although we observed more IL-17A protein in the BAL of Il-1rn−/− mice, most prominently 48 hours after LPS exposure, we viewed these results with caution, because the values were at the lower limits of detection for the ELISA assay (Figure E7A). Figure 7A demonstrates our subsequent confirmation that an increase in Il-17a mRNA occurred in the lungs of Il-1rn−/− mice, most notably at 24 and 48 hours. This pattern of expression is consistent with the role of IL-17A in the maintenance (rather than initiation) of an immune response (36).
Figure 7.
IL-17A expression and IL-17A blockade in Il-1rn−/− mice. (A) Relative expression of Il-17a mRNA (measured by real-time PCR) in Il-1rn−/− mice overall, and at 24 hours (*P < 0.05) and 48 hours (*P < 0.01) after exposure to LPS, compared with littermate control mice (Il-1rn+/+). RQ, relative quantity. (B) Representative dot plots illustrate the number of γδ T cells positive for IL-17A in the lungs of Il-1rn−/− mice, relative to (C) C57BL/6 mice, 48 hours after LPS (n = 4–6 mice per time point). (D) BAL neutrophil counts in Il-1rn−/− mice pretreated 24 hours before exposure to LPS with 100 μg intraperitoneal IL-17A monoclonal antibody (mAb). (E) Lung mRNA G-csf concentrations, 48 hours after exposure to LPS in Il-1rn−/− mice pretreated with 100 μg intraperitoneal isotype control antibody (Ab) (n = 6 mice per time point).
In addition, we examined the expression of other IL-17 family members, as well as the related cytokines IL-22 and IL-23, after LPS exposure in the lungs of Il-1rn−/− mice according to real-time PCR. As seen in Figure E7B, significantly greater Il-17f RNA expression was observed overall, particularly at 48 hours. A mild decrease was evident in Il-17c expression in Il-1rn−/− mice, although this was only significant at 0 hours, as seen in Figure E7C. Figures E7D and E7E illustrate that no differences were observed in the expression of IL-23a or IL-22. Moreover, no difference in relative lung RNA expression was detected in the related, but much less well studied, IL-17 ligands, including Il-17b, Il-17d, or Il-17e, or in the receptor for IL-17E, Il-17rb, between Il-1rn−/− and wild-type mice (data not shown).
Because IL-17A is predominately expressed by lymphoid cells, we determined which lineage expressed intracellular IL-17A. The primary cellular source of the intracellular IL-7A ligand in both wild-type and Il-1rn−/− lungs was γδ T cells. A significant increase overall, and most notably at 48 hours after exposure to LPS, was evident in the percentage of IL-17A+γδ T cells in the lungs of Il-1rn−/− compared with wild-type mice (representative dot plots are shown in Figures 7B, 7C, and E8A). No differences, however, were observed in the percentage of TCRβ+IL-17A+ cells in the lungs of Il-1rn−/− mice compared with C57BL/6 mice, at baseline or 48 hours after LPS exposure (Figures E8G and E8H). Notably, IL-17A was also not expressed intracellularly in the lungs of either group of mice in F4–80+ cells (macrophages) or GR1+ cells (neutrophils) at 0 or 48 hours after exposure to LPS (Figures E8C–E8F).
Since the greatest increase in Il-1rn−/− murine lung expression occurred in IL-17A, and IL-17A is known to regulate the expression of the two cytokines (G-CSF and CXCL1) that our data suggest play a role in inducing pulmonary inflammation in Il-1rn−/− mice, we anticipated that IL-17 blockade would limit the ALI attributable to LPS (13). Pretreatment with IL-17A mAb before exposure to LPS (versus isotype control antibody) decreased the BAL neutrophil count and lung G-csf mRNA expression 48 hours after exposure to LPS (Figures 7D and 7E). A similar trend toward decreased concentrations of BAL and plasma G-CSF was observed, although it was not statistically significant (Figures E9A and E9B). IL-17A mAb administration did not affect the total BAL WBCs (Figure E9C), peripheral cell counts (data not shown), or bone marrow composition (Figures E9D and E9E).
Discussion
The precise mechanisms by which the IL-1 pathway influences the development of ALI remain elusive, despite extensive study. Here, we show that after exposure to aerosolized LPS, significant differences are evident in BAL cell count, protein concentration, and histology in mice lacking essential components of the IL-1 pathway. We also report that alterations in neutrophil influx are associated with differing BAL cytokine concentrations, particularly CXCL1. Furthermore, our work suggests that the involvement of IL-1 in hematopoiesis (likely via the IL-17A/G-CSF axis) may affect levels of systemic inflammation, and delay the resolution of lung injury.
In loss of function experiments, we hypothesized that the deletion of IL-1R1 would attenuate the intensity of the pulmonary inflammatory response. Consistent with this supposition, we found a significant reduction in BAL cell counts of Il-1r1−/− mice, particularly early (at 4 h) and later (at 48 h) after LPS exposure, as well as an overall decrease in BAL protein, a gross estimation of capillary permeability. Concentrations of the chemoattractants CXCL1, CXCL2, and CXCL5 were decreased at 4 hours in Il-1r1−/− murine BAL, and may contribute to this early, attenuated BAL neutrophil accumulation. The early decreases in BAL neutrophil numbers and chemokine protein concentrations at 4 hours occurred without evidence of concomitant increased IL-1 agonist protein concentrations in the BAL (Figure E1A). We speculate this represents scavenging of small amounts of released cytokine by cognate receptors (37). Furthermore, we hypothesize that lower BAL neutrophil counts at 48 hours may be related to decreased G-CSF expression (providing less stimulus for granulopoiesis), a consequence with potentially delayed effects on BAL neutrophil numbers. To our knowledge, the role of IL-1R1 in both the initiation and resolution phases of lung inflammation has not been previously described. The detailed pattern of cellular and cytokine accumulation that we describe may serve to unify contradictory data regarding a role of IL-1R1 in rodent models of acute and chronic lung diseases (38–41).
Although Il-1r1−/− mice exhibited an attenuated inflammatory response to LPS, Il-1rn−/− mice demonstrated increased BAL total WBCs and neutrophils, predominately at later time points (i.e., 36 and 48 hours). Likewise, we found evidence of increased alveolar permeability and unresolved histologic inflammation in the lungs of Il-1rn−/− mice at 48 hours. We associate some of these findings with the elevated BAL CXCL1 protein observed in Il-1rn−/− mice after exposure to LPS, which notably decreased when these mice were pretreated with rhIL-1RA.
In addition to increased BAL cell counts, we observed that Il-1rn−/− mice also demonstrated higher circulating peripheral total WBCs and neutrophil counts later after exposure to LPS. To gain further insights into the hematopoietic system of Il-1rn−/− mice, we analyzed their baseline bone marrow composition and discovered a greater percentage and absolute number of cells of neutrophil lineage. This novel observation led us to speculate that the expanded neutrophil compartment in Il-1rn−/− bone marrow may provide a larger reservoir of cells readily available for recruitment during injury. The altered bone marrow composition may also reflect a chronically inflamed state, as suggested by the lower body weights and increased incidence of inflammatory skin and joint lesions in Il-1rn−/− mice (35).
Our main findings of increased neutrophils in the BAL, peripheral blood, and bone marrow of Il-1rn−/− mice directed us to focus further studies on cytokines regulating hematopoiesis. Higher concentrations of G-CSF protein in both BAL and plasma were found in Il-1rn−/− mice, particularly 1–2 days after exposure to LPS. We speculate that increased G-CSF contributes to higher neutrophil counts via several mechanisms. G-CSF stimulates bone marrow progenitors to speed the development of neutrophils, which supplies more cells to the peripheral circulation and, we propose, renders more neutrophils accessible for rapid recruitment to the lungs. In addition, the elevated G-CSF in the BAL of Il-1rn−/− mice may minimize the apoptosis of those neutrophils, effectively increasing the total number by decreasing neutrophil death (33, 42). G-CSF also induces granulopoiesis, an effect that may exert delayed consequences and explain why increases in BAL neutrophil counts are most prominent in Il-1rn−/− mice at later time points (33, 43).
In addition to its proinflammatory properties, G-CSF has many other functions, including activity as an anti-inflammatory molecule by inducing the expression of IL-1RA (44, 45). This increase in IL-1RA not only limits the activation of the IL-1 pathway, but may prolong the maintenance of some granulocyte progenitors during the G0/G1 phase (46). These arrested cells could then serve as a reservoir to replenish the bone marrow after the acute insult subsides, and provide a mechanism to restore homeostasis. In addition to G-CSF inducing IL-1RA, IL-1RA was shown to decrease G-CSF further (34). We confirmed this in our own model, when we pretreated Il-1rn−/− mice with rhIL-1RA before LPS exposure, and found a resultant reduction in the expression of G-CSF.
Although both LPS and IL-1 may stimulate G-CSF production, IL-17A appears to enhance G-CSF expression in the lungs of Il-1rn−/− mice. Previously, IL-17A was shown to increase in C57BL/6 murine BAL after LPS exposure. However, IL-17A was not specifically associated with G-CSF regulation (29). IL-17A may be produced by several families of lymphocytes as well as myeloid cells, and may respond, in part, to IL-1 (13, 14). We found the predominant source of IL-17A to be a subset of T cells (i.e., γδ T cells), and at the time points we examined, no significant intracellular expression of IL-17A was evident in myeloid cells present in the lungs. When induced, IL-17A production may serve to sustain an already initiated inflammatory response (33, 36). This role in the maintenance of inflammation seems especially plausible in the lungs of Il-1rn−/− mice, where we found evidence of delayed resolution of inflammation, coincident with elevated concentrations of IL-17A.
To study the potential role of IL-17A as an important inducer of G-CSF and neutrophil recruitment in our Il-1rn−/− mice, we administered IL-17A mAb before LPS exposure (21), and discovered a resultant decrease in BAL neutrophil counts and G-CSF mRNA expression in the lung. This is consistent with previous reports showing that IL-17A is important in the pathogenesis of joint inflammation in Il-1rn−/− mice (47). Our findings are also consistent with data from other murine model systems that cited the key role of IL-17A in related inflammatory lung diseases (48, 49). Our data are, to the best of our knowledge, novel because we demonstrate that mice deficient in sIL-1RA exhibit an increased expression of IL-17A in the lung, which is likely involved in the regulation of the pulmonary (and to some degree, systemic) expression of G-CSF and resultant inflammation.
As with any knockout mouse model, animals may not reflect the subtle changes in gene expression that can occur in humans possessing polymorphisms in the IL-1RN gene. During the past few years, however, a complete deficiency state in humans has been described in which humans lacking IL-1RA develop a syndrome known as deficiency of the interleukin-1 receptor antagonist (DIRA). DIRA is a rare condition characterized by sterile osteomyelitis, periositis, and pustulosis, in the absence of infection. These patients respond to treatment with a recombinant human form of IL-1RA. Notably, patients with DIRA demonstrated a markedly increased expression of IL-17A in their cutaneous lesions as well as higher numbers of circulating Th17 cells, further underscoring the potential interactions between IL-1RA and IL-17A in human disease (50).
In conclusion, we show that mice lacking IL-1 receptor–1 develop decreased lung inflammation after exposure to LPS in two phases, likely mediated by differential cytokine expression. Moreover, our data indicate that the increased lung inflammation observed in Il-1rn−/− mice after LPS exposure may be largely attributable to a delay in the resolution of injury, because of elevated concentrations of CXCL1 and G-CSF. We propose that IL-1RA normally acts to limit acute lung inflammation by suppressing IL-17A concentrations, thereby providing less subsequent stimulus for G-CSF. Moreover, with relatively decreased G-CSF expression, we postulate a reduced induction of granulopoiesis during the acute insult, and a more rapid resolution of lung injury. Further study is needed to determine if similar mechanisms are incited during acute lung injury in humans, but blockade of IL-17A (potentially via the alteration of IL-1RA) offers an intriguing potential new therapeutic strategy for ALI.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants T32-HD060556 (G.S.W.), R01-HLO68876 (G.S.W.), R01-HLI05834 (G.S.W.), T32-HL007586, K12-HL090021, and 2KL2RR024132, and by a William Dutch Dyson Research Award.
Author Contributions: K.M.H., Y.L., and G.S.W. were involved in the conception, delineation of hypotheses, and design of the study. K.M.H., Y.L., J.M., R.C.M., J.E.H., N.D., and G.S.W. were involved in the acquisition of data or analysis and interpretation of such information. K.M.H. and G.S.W. were involved in writing the article or were substantially involved in its revision before submission.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0104OC on May 16, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gabay C, Palmer G. Mutations in the IL1RN locus lead to autoinflammation. Nat Rev Rheumatol 2009;5:480–482 [DOI] [PubMed] [Google Scholar]
- 2.Arnalich F, Lopez-Maderuelo D, Codoceo R, Lopez J, Solis-Garrido LM, Capiscol C, Fernandez-Capitan C, Madero R, Montiel C. Interleukin-1 receptor antagonist gene polymorphism and mortality in patients with severe sepsis. Clin Exp Immunol 2002;127:331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy H, Murphy A, Zou F, Gerard C, Klanderman B, Schuemann B, Lazarus R, García KC, Celedón JC, Drumm M, et al. IL1B polymorphisms modulate cystic fibrosis lung disease. Pediatr Pulmonol 2009;44:580–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasakova M, Sterclova M, Kolesar L, Slavcev A, Pohunek P, Sulc J, Striz I. Cytokine gene polymorphisms and BALF cytokine levels in interstitial lung diseases. Respir Med 2009;103:773–779 [DOI] [PubMed] [Google Scholar]
- 5.Meyer NJ, Sheu CC, Li M, Chen F, Gallop R, Localio AR, Bellamy S, Kaplan S, Lanken P, Fuchs BF, et al. IL1RN polymorphism is associated with lower risk of acute lung injury in two separate at-risk populations [abstract]. Am J Respir Crit Care Med 2010;181:A1023 [Google Scholar]
- 6.Donnelly SC, Strieter RM, Reid PT, Kunkel SL, Burdick MD, Armstrong I, Mackenzie A, Haslett C. The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann Intern Med 1996;125:191–196 [DOI] [PubMed] [Google Scholar]
- 7.Fiocco U, Vezzu M, Cozzi L, Todesco S. IL-1Ra (recombinant human IL-1 receptor antagonist) in the treatment of rheumatoid arthritis: the efficacy. Reumatismo 2004;56:62–73 [DOI] [PubMed] [Google Scholar]
- 8.Fisher CJ, Jr, Dhainaut JA, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome: results from a randomized, double-blind, placebo-controlled trial. JAMA 1994;271:1836–1843 [PubMed] [Google Scholar]
- 9.Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol 2010;6:232–241 [DOI] [PubMed] [Google Scholar]
- 10.Dinarello C. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009;27:519–550 [DOI] [PubMed] [Google Scholar]
- 11.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal 2010;3:cm1. [DOI] [PubMed] [Google Scholar]
- 12.Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology 2011;134:8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 2010;129:311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brodlie M, Lordan J, Ward C. Can cells other than Th17 lymphocytes be important sources of IL-17 in the lungs? Thorax 2011;66:1096, author reply 1096–1097 [DOI] [PubMed] [Google Scholar]
- 15.Balamayooran G, Batra S, Fessler MB, Happel KI, Jeyaseelan S. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am J Respir Cell Mol Biol 2010;43:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang KY, Arcaroli JJ, Abraham E. Early alterations in neutrophil activation are associated with outcome in acute lung injury. Am J Respir Crit Care Med 2003;167:1567–1574 [DOI] [PubMed] [Google Scholar]
- 17.Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal 2011;23:1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mei J, Liu Y, Dai N, Hoffmann C, Hudock KM, Zhang P, Guttentag SH, Kolls JK, Oliver PM, Bushman FD, et al. CXCR2 and CXCL5 regulate the IL-17/G-CSF axis and neutrophil homeostasis in mice. J Clin Invest 2012;122:974–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony–stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 2001;194:519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Mei J, Gonzales L, Yang G, Dai N, Wang P, Zhang P, Favara M, Malcolm KC, Guttentag S, et al. IL-17A and TNF-alpha exert synergistic effects on expression of CXCL5 by alveolar Type II cells in vivo and in vitro. J Immunol 2011;186:3197–3205 [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Feng Y, Yang K, Li Q, Ye L, Han L, Wan H. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS Immunol Med Microbiol 2011;61:179–188 [DOI] [PubMed] [Google Scholar]
- 22.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol 2001;25:335–340 [DOI] [PubMed] [Google Scholar]
- 23.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol 2009;183:5301–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol 1998;16:27–55 [DOI] [PubMed] [Google Scholar]
- 25.Hudock KM, Liu Y, Worthen GS. Il-1 receptor knockout mice have an increased early inflammatory response to acute LPS inhalation. Am J Respir Crit Care Med 2010;181:A2770 [abstract] [Google Scholar]
- 26.Hudock KM, Liu Y, Worthen GS. Interleukin-1 receptor antagonist knockout mice have an increased acute inflammatory response in the lung after LPS. Am J Respir Crit Care Med 2011;183:A1089 [abstract] [Google Scholar]
- 27.Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun 2004;72:7247–7256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nick JA, Young SK, Arndt PG, Lieber JG, Suratt BT, Poch KR, Avdi NJ, Malcolm KC, Taube C, Henson PM, et al. Selective suppression of neutrophil accumulation in ongoing pulmonary inflammation by systemic inhibition of p38 mitogen–activated protein kinase. J Immunol 2002;169:5260–5269 [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol 2003;170:4665–4672 [DOI] [PubMed] [Google Scholar]
- 30.Weber A, Wasiliew P, Kracht M. Interleukin-1beta (IL-1beta) processing pathway. Sci Signal 2010;3:cm2 [DOI] [PubMed] [Google Scholar]
- 31.Mei J, Liu Y, Dai N, Favara M, Greene T, Jeyaseelan S, Poncz M, Lee JS, Worthen GS. CXCL5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity 2010;33:106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston CJ, Finkelstein JN, Gelein R, Oberdorster G. Pulmonary cytokine and chemokine mRNA levels after inhalation of lipopolysaccharide in C57BL/6 mice. Toxicol Sci 1998;46:300–307 [DOI] [PubMed] [Google Scholar]
- 33.Panopoulos AD, Watowich SS. Granulocyte colony–stimulating factor: molecular mechanisms of action during steady state and “emergency” hematopoiesis. Cytokine 2008;42:277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy SM, Borch RF. IL-1β mediates diethyldithiocarbamate-induced granulocyte colony–stimulating factor production and hematopoiesis. Exp Hematol 1999;27:210–216 [DOI] [PubMed] [Google Scholar]
- 35.Hirsch E, Irikura VM, Paul SM, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci USA 1996;93:11008–11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maione F, Paschalidis N, Mascolo N, Dufton N, Perretti M, D’Acquisto F. Interleukin 17 sustains rather than induces inflammation. Biochem Pharmacol 2009;77:878–887 [DOI] [PubMed] [Google Scholar]
- 37.Rot A. Chemokine patterning by glycosaminoglycans and interceptors. Front Biosci 2010;15:645–660 [DOI] [PubMed] [Google Scholar]
- 38.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF, Lagente V, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest 2007;117:3786–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brass DM, Hollingsworth JW, Fessler MB, Savov JD, Maxwell AB, Whitehead GS, Burch LH, Schwartz DA. The IL-1 Type 1 receptor is required for the development of LPS-induced airways disease. J Allergy Clin Immunol 2007;120:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank JA, Pittet J, Wray C, Matthay MA. Protection from experimental ventilator-induced acute lung injury by IL-1 receptor blockade. Thorax 2008;63:147–153 [DOI] [PubMed] [Google Scholar]
- 41.Moreland JG, Fuhrman RM, Wohlford-Lenane CL, Quinn TJ, Benda E, Pruessner JA, Schwartz DA. TNF-alpha and IL-1 beta are not essential to the inflammatory response in LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol 2001;280:L173–L180 [DOI] [PubMed] [Google Scholar]
- 42.Droemann D, Hansen F, Aries SP, Braun J, Zabel P, Dalhoff K, Schaaf B. Neutrophil apoptosis, activation and anti-inflammatory cytokine response in granulocyte colony–stimulating factor–treated patients with community-acquired pneumonia. Respiration 2006;73:340–346 [DOI] [PubMed] [Google Scholar]
- 43.Hannen M, Banning U, Bonig H, Kim YM, Shin DI, Lorenz I, Seeger K, Korholz D. Cytokine-mediated regulation of granulocyte colony–stimulating factor production. Scand J Immunol 1999;50:461–468 [DOI] [PubMed] [Google Scholar]
- 44.Schwabe M, Hartert A, Bertz H, Finke J. Treatment with granulocyte colony–stimulating factor increases interleukin-1 receptor antagonist levels during engraftment following allogeneic stem-cell transplantation. Eur J Clin Invest 2004;34:759–765 [DOI] [PubMed] [Google Scholar]
- 45.Pajkrt D, Manten A, van der Poll T, Tiel-van Buul MMC, Jansen J, Wouter ten Cate J, van Deventer S. Modulation of cytokine release and neutrophil function by granulocyte colony–stimulating factor during endotoxemia in humans. Blood 1997;90:1415–1424 [PubMed] [Google Scholar]
- 46.Zhang J, Xiang D, Zhu S, Mao W, Lu H, Wu M, Wang Q, Yu Y, Herbst KD, Han W. Interleukin 1 receptor antagonist inhibits normal hematopoiesis and reduces lethality and bone marrow toxicity of 5-fluouracil in mouse. Biomed Pharmacother 2009;63:501–508 [DOI] [PubMed] [Google Scholar]
- 47.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA 2003;100:5986–5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med 2011;184:252–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Traves SL, Donnelly LE. Th17 cells in airway diseases. Curr Mol Med 2008;8:416–426 [DOI] [PubMed] [Google Scholar]
- 50.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, Laxer R, Tedgård U, Cowen EW, Pham T, et al. An autoinflammatory disease with deficiency of the interleukin-1–receptor antagonist. N Engl J Med 2009;360:2426–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.