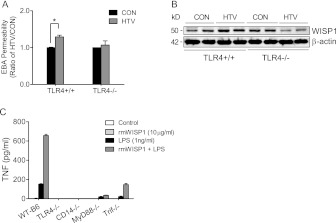
Figure 7.
WISP1 contribution to VILI is dependent on TLR4 signaling pathway. (A) HTV increased EBA permeability in TLR4+/+ mice compared with CON, but did not increase EBA permeability in TLR4−/− mice after 4 hours of mechanical ventilation. Values are means (±SEM) (n = 3 mice/group). Significant difference from control (*P < 0.05), as determined by two-way ANOVA followed by Bonferroni’s multiple comparisons. (B) WISP1 protein expression was determined in strain-matched control (TLR4+/+) and TLR4 gene–targeted (TLR4−/−) mice after 4 hours of HTV ventilation. HTV increased WISP1 protein expression in TLR4+/+ mice, but not in TLR4−/− mice compared with CON. β-actin was the loading control. Data are representative of three tests. (C) rmWISP1 enhanced LPS-induced TNF release in peritoneal macrophages via CD14–TLR4 signaling. Peritoneal macrophages were seeded in 96-well plates at a density of 6 × 104 cells/well for 24 hours before treating with indicated concentrations of rmWISP1, LPS, and LPS + rmWISP1 for 22 hours, and TNF release in the culture medium was measured by ELISA. rmWISP1, by itself, caused minimal TNF release. rmWISP1, however, enhanced LPS-induced TNF release in peritoneal macrophages from C57BL/6J strain-matched control (WT-B6), but did not amplify LPS-induced TNF release in macrophages obtained from TLR4 gene–targeted (TLR4−/−) mice or CD14 gene–targeted (CD14−/−) mice. In addition, rmWISP1-enhanced LPS-induced TNF release was inhibited more in macrophages isolated from myeloid differentiation primary response gene 88 gene–targeted (MyD88−/−) mice than in macrophages from TLR adaptor molecule 1 (TICAM1; a.k.a., TRIF) mutant (TRIF−/−) mice. Values are means (±SEM) from three independent experiments in duplicate wells.