Abstract
Increased expression of tumor suppressor protein p53 and of plasminogen activator inhibitor (PAI)-1 is associated with cigarette smoke (CS) exposure–induced lung epithelial injury. p53 induces PAI-1 through mRNA stabilization in lung epithelial cells. However, it is unclear how this process affects lung epithelial damage. Here, we show that CS induces p53 and PAI-1 expression and apoptosis in cultured Beas2B and primary alveolar type (AT)II cells. CS exposure augmented binding of p53 protein with PAI-1 mRNA. Inhibition of p53 from binding to PAI-1 mRNA through expression of p53-binding 70 nt PAI-1 mRNA 3′UTR sequences suppressed CS-induced PAI-1 expression. Treatment of Beas2B cells with caveolin-1 scaffolding domain peptide (CSP) suppressed p53 expression and p53–PAI-1 mRNA interaction. These changes were associated with parallel inhibition of CS-induced PAI-1 expression and apoptosis in Beas2B cells. Wild-type mice exposed to passive CS likewise show augmented p53 and PAI-1 with parallel induction of ATII cell apoptosis, whereas mice deficient for p53 or PAI-1 expression resisted apoptosis of ATII cells. CSP suppressed CS-induced ATII cell apoptosis in wild-type mice and abrogated p53–PAI-1 mRNA interaction with parallel inhibition of p53 and PAI-1 expression. The protection against ATII cell apoptosis by CSP involves inhibition of passive CS-induced proapoptotic Bax and Bak expression and restoration of the prosurvival proteins Bcl-XL. These observations demonstrate that inhibition of p53 binding to PAI-1 mRNA 3′UTR attenuates CS-induced ATII cell apoptosis. This presents a novel link between p53-mediated PAI-1 expression and CS-induced ATII cell apoptosis.
Keywords: cigarette smoke, caveolin-1 scaffolding domain peptide, ATII cell apoptosis, lung epithelial injury
Clinical Relevance
These studies provide novel information about how p53 and PAI-1 expression induced by passive cigarette smoke exposure contributes to ATII cell apoptosis and lung injury.
Active cigarette smoking or exposure to passive cigarette smoke (CS) contributes to a debilitating clinical condition associated with pulmonary emphysema, chronic bronchitis, and chronic obstructive pulmonary disease (COPD) (1, 2). COPD is the fourth leading cause of death in the United States (3, 4) and is characterized by lung epithelial injury and remodeling, contributing to irreversible airflow limitation (5). Defective fibrinolysis and alveolar fibrin deposition, due to a disproportionate increase in plasminogen activator inhibitor (PAI)-1 and epithelial cell apoptosis, are often associated with multiple forms of inflammatory lung diseases (6, 7). Airway and alveolar epithelial cell apoptosis, as a consequence of increased p53 expression resulting from DNA damage, occur frequently in lung injuries, including CS exposure–induced lung injury (8). Augmented PAI-1 and p53 expression is observed after CS-induced alveolar epithelial cell damage. Plasma PAI-1 levels are also increased in cigarette smokers compared with nonsmokers (9).
We previously reported that p53 induces PAI-1 expression through post-transcriptional PAI-1 mRNA stabilization. The process involves p53 interaction through its C-terminal region with a specific 70 nt sequence present in the 3′UTR of PAI-1 mRNA (10). We also showed that inhibition of PAI-1 or p53 expression mitigates bleomycin or CS extract (CSE)-induced Beas2B cell apoptosis in vitro. Others have also reported the requirement of PAI-1 for p53-induced fibroblast senescence (11). However, it is unclear how the intricate link between defective alveolar fibrinolysis and epithelial cell damage contributes to lung injury. Here we show that p53 induction, due to DNA damage after exposure to CS, induces ATII cell apoptosis and that the process involves downstream PAI-1 expression. Preventing CS-induced p53 from binding to PAI-1 mRNA mitigates ATII cell apoptosis in vitro and in vivo, demonstrating that p53 facilitates lung epithelial cell damage through induction of PAI-1 expression.
Materials and Methods
Cells
Human Beas2B cells were cultured as previously described (12). ATII cells were isolated from C57BL/6 mice following the method of Corti and colleagues (13) with minor modifications. The cells were plated on plastic culture dishes precoated with anti–CD-32 and anti–CD-45 antibodies for 2 hours at 37°C. Nonadherent cells were collected, and the purity of ATII cell preparations was assessed by lithium carbonate staining for inclusion bodies.
Preparation of CSE
CSE was prepared using research cigarettes 2R4F from the Tobacco Health Research University of Kentucky (Lexington, KY) by following the method developed by Carp and Janoff (14).
Passive CS Exposure of Mice
All mouse experiments were performed according to the approved protocols under the guidelines of Animal Care and Use Committee of The UT Health Science Center at Tyler. WT, p53-deficient, and PAI-1–deficient mice of C57BL-6 background were bred in our facilities or were purchased from Jackson Laboratories. These mice were exposed to passive CS from 40 cigarettes over a 2-hour period 5 days per week for 20 weeks (∼ 90 mg/m3 total solid particulates) using a mechanical smoking chamber (Teague Enterprises, Davis, CA). Control mice were exposed to ambient air. Four weeks after initiation of passive CS exposure, the mice were administered an intraperitoneal injection of 18.75 mg/kg body wt of caveolin-1 scaffolding domain peptide (CSP) (DGIWKASFTTFTVTKYWFYR) or CP (WGIDKAFFTTSTVTY KWFRY) once a week for the next 4 weeks. Exposure to passive cigarette was continued for another 12 weeks. Mice were killed, and their lungs were used for various analyses.
Immunoblotting and Immunohistochemistry
p53, PAI-1, active (cleaved) and total caspase-3, Bak, Bax, Bcl-XL, phosphorylated and total Akt, and PTEN antigen levels were assessed by Western blotting of isolated ATII cell lysates and confirmed with immunohistochemical analysis (15–17).
Analysis of PAI-1 mRNA Expression
Total RNA from Beas2B and ATII cells treated with CSE, as well as ATII cells isolated from WT mice exposed to CS for 20 weeks, were analyzed for changes in PAI-1 and β-actin mRNA by RT-PCR or Northern blotting (10).
Analysis of PAI-1 mRNA Synthesis and Decay
Beas2B cells treated with PBS or CSE were analyzed for the rate of PAI-1 mRNA synthesis by run-on transcription assay (18). Beas2B cells were treated with PBS or CSE for 9 hours to induce maximum PAI-1 mRNA. The PAI-1 mRNA decay was determined by 5,6-dichlorobenzimidazole riboside chase experiments (10).
Analysis of Changes in p53 and PAI-1 mRNA Interaction
Beas2B cells exposed to CSE for 24 hours and ATII cells isolated from mice exposed to passive CS for 20 weeks were subjected to chemical cross-linking to immobilize RNA–protein complexes (10). Protein extracts from these cells were precleared with mouse IgG coupled to agarose. The supernatant was immunoprecipitated with anti-p53 antibody. PAI-1 mRNA associated with p53 was analyzed by RT-PCR in the presence of 32P-dCTP. The amplified PCR products were separated on a urea/PAGE and autoradiographed (10).
Statistical Analysis
The differences between two and multiple groups under various experimental conditions were analyzed by Student’s t test and one-way ANOVA, respectively.
Results
CSE Induces p53 and PAI-1 Expression through Post-Transcriptional mRNA Stabilization
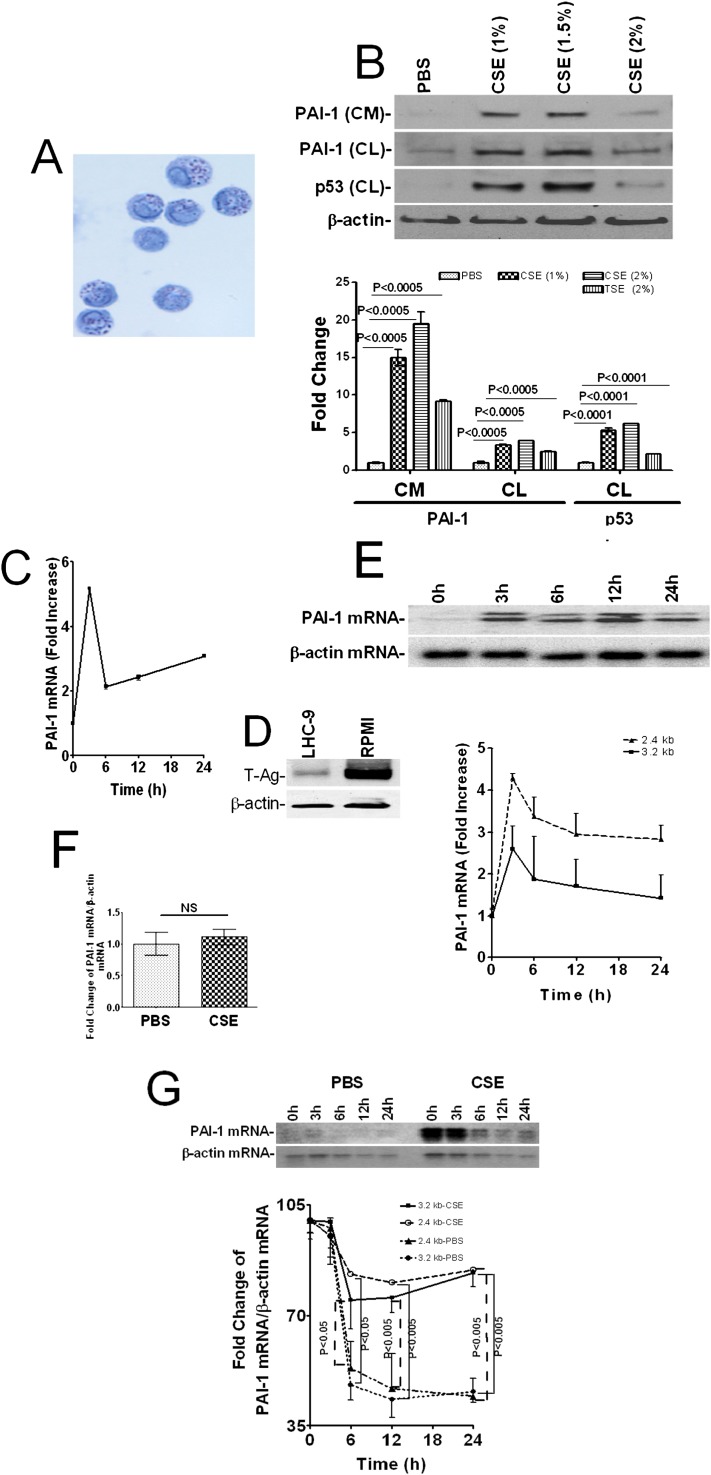
ATII cells isolated from mouse lungs were 90 to 95% pure as confirmed by staining for inclusion bodies (Figure 1A). Immunoblotting of conditioned media and cell lysates of mouse lung ATII cells for PAI-1 and p53 indicated that p53 and PAI-1 proteins were induced after exposure to CSE in a dose-dependent manner, with maximum increase observed at 1.5% CSE (Figure 1B). Therefore, 1.5% CSE was used in all the subsequent in vitro experiments. These responses are consistent with findings in human Beas2B cells (10). We analyzed PAI-1 mRNA by RT-PCR to confirm whether PAI-1 mRNA expression was also increased. In agreement with the protein expression, exposure of mouse ATII cells to CSE induced PAI-1 mRNA in a time-dependent manner, with the maximum effect observed within 3 hours (Figure 1C). PAI-1 expresses 3.2- and 2.4-kb transcripts due to alternate splicing (10). We used Northern blotting to determine whether both spliced variants were induced by CS exposure. Primary ATII cells yielded inadequate amounts of RNA for the analysis by Northern blotting, and Beas2B cells cultured in LHC-9 medium expressed minimum SV40 T-antigen (Figure 1D) and responded by induction of PAI-1–like primary lung epithelial cells, despite being transformed (12, 18). Therefore, we treated Beas2B cells in LHC-9 medium with CSE for 0 to 24 hours and analyzed for spliced variants of PAI-1 mRNA by Northern blotting. CSE induced the 2.4- and 3.2-kb components of PAI-1 mRNA and peaked between 3 to 12 hours after exposure (Figure 1E).
Figure 1.
Induction of p53 and PAI-1 expression by mouse and human lung epithelial cells after cigarette smoke (CS) exposure. (A) Primary alveolar type (AT)II cells isolated from the lungs of C57BL/6 mice were subjected to cytospin and stained with lithium carbonate for inclusion bodies. (B) ATII cells treated with 0 to 2% of CS extract (CSE) for 24 hours in alveolar epithelial cell culture media without antibiotic. The conditioned media (CM) was analyzed for plasminogen activator inhibitor (PAI)-1. The cell lysates (CL) were tested for p53, PAI-1, and β-actin expression by Western blotting. Fold changes in the densities of individual PAI-1 bands from the Western blot of CM are presented as a bar graph without normalization. The densities of the PAI-1 and p53 bands in the CL were normalized to β-actin controls run on the same gel. (C) Total RNA isolated from mouse ATII cells exposed to CSE (1.5%) for 0 to 24 hours were tested for PAI-1 and β-actin mRNA expressions by RT-PCR in the presence of 32P-labeled dCTP. The fold changes in densities of individual bands were quantitated and normalized against the corresponding β-actin mRNA levels in the same sample. (D) Beas2B cells cultured in LHC-9 and RPMI media containing 10% FBS for several days were lysed, and the lysates were tested for expression of SV40 T-antigen by Western blotting. The same membrane was stripped and blotted for β-actin to assess loading equality. (E) Total RNA isolated from Beas2B cells was analyzed for PAI-1 and β-actin mRNAs by Northern blotting using 32P-labeled cDNA probes. The densities of individual bands for the 2.4- and 3.2-kb components of PAI-1 mRNA were normalized with β-actin mRNA loading control. Fold changes in PAI-1 mRNA at 3, 6, 12, and 24 hours versus 0 hours are presented as a line graph. (F) Extracts of the isolated nuclei from Beas2B cells treated with PBS or CSE as above for 3 hours were analyzed for rate of PAI-1 mRNA synthesis by run-on transcription assay. The densities of individual bands were quantitated after normalization with β-actin controls and are shown as a bar graph. NS = differences were not statistically significant. (G) Beas2B cells were treated with PBS or CSE as above for 9 hours to induce maximum PAI-1 mRNA expression. Total RNA was isolated at various time points (0–24 h) after inhibiting ongoing transcription by adding 5,6-dichlorobenzimidazole riboside (20 μg/ml) to the same media. The decay of PAI-1 mRNA was analyzed by Northern blotting, and the densitometric scanning of individual bands is shown as a line graph, where the mRNA level at 0 hours for each treatment is calculated as 100%. All experiments were repeated at least three to four times, and representative results are illustrated.
The inability of CSE to induce PAI-1 in Beas2B cells after blockade of p53 expression (10) and p53 involvement in PAI-1 promoter activation in osteosarcoma cells (19) suggest that increased PAI-1 mRNA transcription by CS-induced p53 contributes to PAI-1 expression. However, results of run-on transcription experiments indicated that the rate of PAI-1 mRNA synthesis in Beas2B cells treated with CSE was comparable to those cells exposed to vehicle (PBS) alone (Figure 1F), thus excluding its involvement in augmenting PAI-1 expression. p53 does not enhance PAI-1 mRNA translation in Beas2B cells; however, it stabilizes PAI-1 mRNA (10). This led us to believe that reduced PAI-1 mRNA decay could contribute to induction of PAI-1 by CS. Results of transcriptional chase experiments indicated that PAI-1 mRNA is highly labile under normal conditions; however, exposure to CSE stabilized the 2.4- and 3.2-kb variant transcripts of PAI-1 mRNA (Figure 1G).
CS Exposure Increases p53 Interaction with the PAI-1 mRNA
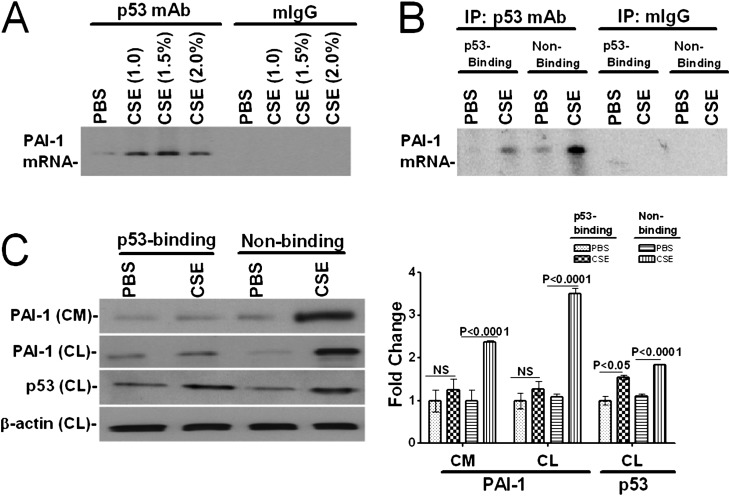
p53 binds to and induces PAI-1 expression through stabilization of PAI-1 mRNA (10). Therefore, we postulated that increased p53 and PAI-1 mRNA binding could induce PAI-1 expression through post-transcriptional mRNA stabilization after exposure to CS. To test this possibility, we immunoprecipitated p53 proteins from the lysates of Beas2B cells treated with PBS or CSE in PBS and analyzed PAI-1 mRNA associated with the p53 protein. We found that treatment of Beas2B cells with CSE increased p53 interaction with the PAI-1 mRNA in a dose-dependent fashion (Figure 2A). Because p53 binds to a 70 nt 3′UTR sequence, we expressed this sequence (10) in Beas2B cells and exposed them to CSE. Expression of the p53-binding PAI-1 mRNA 3′UTR sequence suppressed coprecipitation of PAI-1 mRNA with the p53 proteins due to competitive inhibition, whereas expression of control sequences failed to interfere (Figure 2B). Western blot analysis of the conditioned media and cell lysates for PAI-1 proteins further confirmed that blockade of the p53–PAI-1 mRNA interaction by expression of the p53-binding 3′UTR sequence suppressed CSE-induced PAI-1 expression (Figure 2C). p53 expression was increased in cells exposed to CSE irrespective of being transduced with the p53-binding or control sequences. Lentiviral expression of p53-binding, but not control sequences, in primary human small airway epithelial cells also suppressed CSE-induced PAI-1 expression (data not shown). These results demonstrate that augmented p53 binding with PAI-1 mRNA 3′UTR contributes to increased PAI-1 expression after CSE-induced injury in lung epithelial cells.
Figure 2.
CSE induces interaction of p53 protein with PAI-1 mRNA in lung epithelial cells. (A) Beas2B cells were treated with PBS or CSE (1–2%) for 12 hours at 37°C in LHC-9 media. The cell lysates were immunoprecipitated, total RNA was isolated from the immune complexes, and associated PAI-1 mRNA was amplified by RT-PCR using specific primers in the presence of 32P-labeled dCTP. PAI-1 cDNA products were separated by urea/PAGE, dried, and autoradiographed as we described recently (38). (B) Competitive inhibition of p53 binding to endogenous PAI-1 mRNA by overexpressing p53 binding PAI-1 mRNA 3′UTR sequence. Beas2B cells expressing p53 binding 70 nt PAI-1 mRNA 3′UTR sequence (p53-Binding) or the corresponding non-p53 binding (Non-Binding) control sequences of PAI-1 mRNA (10) were treated with PBS or CSE for 12 hours. The lysates were immunoprecipitated, and the associated immune complexes were analyzed by RT-PCR as described in A. (C) Inhibition of p53-binding to endogenous PAI-1 mRNA by expressing p53 binding PAI-1 mRNA 3′UTR sequence inhibits CS-induced PAI-1 expression. Beas2B cells infected with lentivirus constructs expressing p53-binding or nonbinding control sequences as described in B. Twenty-four hours later, the CM was analyzed for PAI-1 expression, and the CL were tested for PAI-1, p53, and β-actin expression by Western blotting. The bar graph represents the fold change in the individual densities of PAI-1 in the CM. The densities of PAI-1 and p53 bands in the CL were presented as fold change after normalization with corresponding β-actin controls. Experiments were repeated at least three times, and representative results are illustrated.
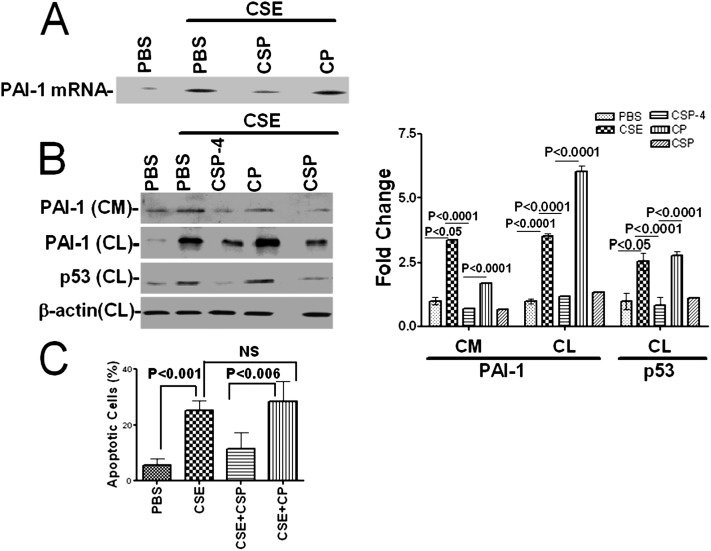
Increased p53 and PAI-1 expression in alveolar and airway epithelial cells contributes to lung epithelial damage and alveolar fibrin deposition (20). Inhibition of p53 expression by siRNA (10) or competitive inhibition of p53 and PAI-1 mRNA interactions by the expression of PAI-1 mRNA 3′UTR sequences suppresses CSE-induced PAI-1 expression in lung epithelial cells. These findings suggest that interruption of the p53 and PAI-1 mRNA interaction could prevent lung epithelial damage and fibrin deposition in vivo. However, limitations of lentiviral transduction of the PAI-1 3′UTR sequence to inhibit p53 from binding to PAI-1 mRNA in vivo prompted us to explore an alternate approach. We previously reported that activation of β1-integrin by antibody ligation or treatment with uPA (20 nM) inhibits p53 expression induced by lung epithelial cells (12). CSP treatment activates β1-integrin and mimics the inhibitory effects of uPA exposure and anti–β1-integrin antibody ligation combined (20). In addition, unlike uPA or the anti–β1-integrin antibody, CSP lacks enzymatic activity and globular conformation and is therefore better suited for in vivo intervention. We first treated Beas2B cells exposed to CSE with 10 nM CSP to attenuate p53 expression. Our results showed that exposure of cells to CSP suppressed the p53 interaction with PAI-1 mRNA. This was confirmed by immunoprecipitation of p53 and RT-PCR for PAI-1 mRNA compared with cells treated with control peptide containing the scrambled sequence (CP) (Figure 3A). Treatment with CSP or a seven–amino acid deletion fragment of CSP (CSP-4, FTTFTVT) attenuated PAI-1 expression induced by CSE (Figure 3B), However, unlike expression of p53 binding sequences, CSP or CSP-4 treatment also inhibited p53 expression. Flow cytometry analysis of annexin-V and propidium iodide–stained cells further demonstrated that CSP treatment protects Beas2B cells against apoptosis caused by CSE, whereas CP-treated cells exhibit massive apoptosis (Figure 3C).
Figure 3.
Inhibition of CS exposure-induced p53 expression by CSP protects lung epithelial cells against apoptosis. (A) Beas2B cells were treated with PBS or CSE (1.5%) in the presence or absence of CSP (10 nM) or CP for 24 hours at 37°C. The cell lysates were immunoprecipitated with anti-p53 antibody, and the PAI-1 mRNA associated with p53-immune complexes were analyzed by RT-PCR as described in Figure 2A. (B) The CM and CL were tested for PAI-1 and p53 expression by Western blotting. The membranes containing proteins from CL were stripped and developed for β-actin to assess equal loading. The fold change in PAI-1 is presented as a bar graph based on the densities of individual bands in the CM under various treatment conditions versus PBS controls. The fold change in PAI-1 and p53 expression in the CL was presented after normalization of band densities with corresponding β-actin loading controls. (C) The cells were harvested, and apoptosis was determined by flow cytometry after treating with Annexin-V and propidium iodide as we explained elsewhere (12). The percentage of apoptotic cells is presented as a bar graph. NS = not statistically significant.
CSP Inhibits CS Exposure–Induced p53 and PAI-1 Expression in Mouse Lungs
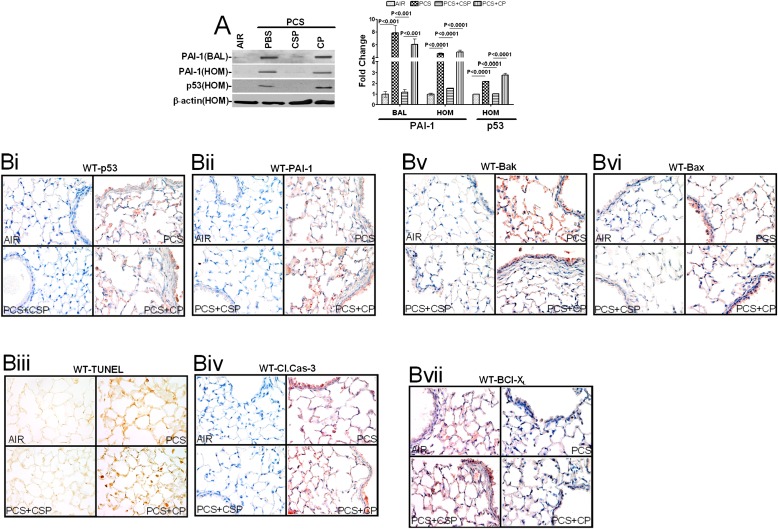
To determine if lung epithelial injury due to CS exposure induces p53 and if p53 plays a pivotal role in the induction of PAI-1 expression in vivo, we exposed WT mice to ambient air or passive CS for 20 weeks. PAI-1 was analyzed in bronchial alveolar lavage (BAL) fluids and lung homogenates, whereas p53 was analyzed in lung homogenates. All of the mice (n = 5 per group) exposed to passive CS showed increased PAI-1 and p53 proteins compared with the control mice. We next sought to determine whether CSP inhibits p53 and PAI-1 expression induced by passive CS exposure. We injected WT mice with or without CSP or CP 4 weeks after initiation of passive CS exposure. At the end of 20 weeks, BAL fluids and lung homogenates were analyzed for changes in p53 and PAI-1. Consistent with the outcomes of Beas2B cells in vitro (Figure 3B), CSP treatment of mice significantly suppressed the expression of p53 and PAI-1 (Figure 4A).
Figure 4.
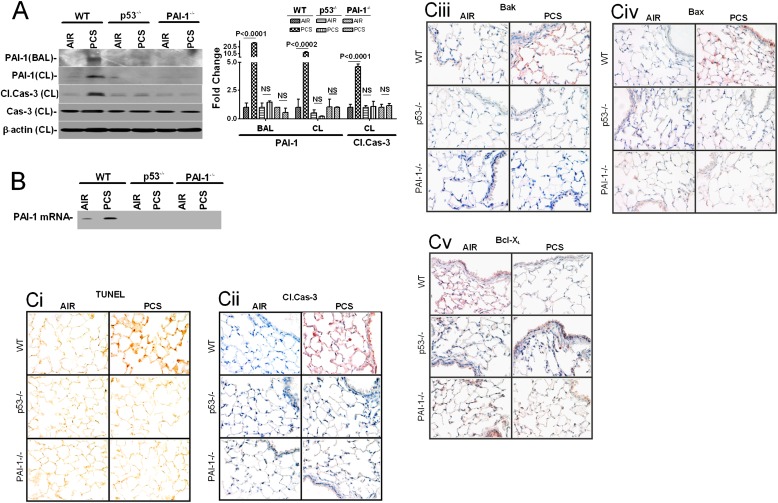
CSP treatment inhibits CS-induced p53 and PAI-1 expressions and apoptosis in wild-type (WT) mouse lung epithelial cells. (A) BAL fluid and lung homogenates (HOM) from mice (n = 5 per group) exposed to ambient air or passive CS for 20 weeks in the presence or absence of CSP or CP were immunoblotted for the changes in PAI-1 and p53 expressions. Membranes containing proteins from the lung homogenates were stripped and analyzed for β-actin to assess equal loading. The fold change in PAI-1 is presented as a bar graph based on densities of individual bands in the BAL fluid under various treatment conditions versus PBS controls. Individual bar represents fold changes in p53, and PAI-1 expression versus air control is shown as a bar graph. (B) Lung sections of WT mice as described in Figure 4A were subjected to immunohistochemical analyses for p53 (Bi) or PAI-1 (Bii), TUNEL staining (Biii), active Caspase-3 (Biv), Bak (Bv), Bax (Bvi), and Bcl-XL (Bvii) to assess changes in the expressions of p53, PAI-1, and pro-apoptotic and anti-apoptotic proteins as well as apoptosis in situ. Experiments were repeated at least three times, and representative results are illustrated.
Histological analysis of lung sections also showed increased staining for p53 (Figure 4Bi) and PAI-1 (Figure 4Bii) antigens after CS exposure. These results are consistent with induction of p53 and PAI-1 expressions by airway and alveolar epithelial cells in response to CS exposure (Figure 1). We then analyzed the lung sections to confirm whether the protection against lung epithelial damage after CSP treatment involves suppression of p53. As expected, treatment with CSP, but not CP, significantly attenuated CS-induced p53 expression in WT mice (Figure 4Bi), suggesting that increased p53 due to passive CS exposure contributes to lung epithelial injury and that protection by CSP was associated with parallel inhibition of p53. CSP treatment also inhibited PAI-1 expression induced by passive CS in WT mice (Figure 4Bii). Parallel with elevated p53 and PAI-1 expression, CS exposure also increased ATII cell apoptosis by about 4-fold, as indicated by the TUNEL-positive (brown) cells (Figure 4Biii) and active caspase-3 antigen (Figure 4Biv) staining of airway and alveolar epithelial cells. Treatment with CSP provided protection against CS-induced lung epithelial cell apoptosis, whereas CP was ineffective against apoptosis. To further understand the contributory mechanism, lung sections were subjected to immunohistochemical (IHC) staining for the Bcl-2 family of proteins, including Bak (Figure 4Bv), Bax (Figure 4Bvi), and Bcl-XL (Figure 4Bvii). Our results show that passive CS induced proapoptotic Bak and Bax but inhibited antiapoptotic Bcl-XL proteins, indicating the involvement of the intrinsic pathway. CSP treatment inhibited passive CS–induced Bak and Bax expression and induced Bcl-XL.
CSP-mediated protection against ATII cell apoptosis during passive CS-exposed lung injury was associated with concurrent inhibition of p53 and PAI-1 expression (Figure 4). This suggests that p53-induced PAI-1 contributes to ATII cell apoptosis. To answer this question, we exposed mice deficient for the expression of p53 or PAI-1 to passive CS for 20 weeks and analyzed the BAL fluids for PAI-1 expression. ATII cells isolated from these mice were tested for PAI-1 and caspase-3 activation to determine if PAI-1 induced by p53 contributes to ATII cell apoptosis. The response of p53- and PAI-1–deficient mice was compared with similarly treated WT mice. As expected, passive CS exposure of WT mice for 20 weeks induced ATII cell apoptosis, as indicated by the robust activation of caspase-3. These changes were associated with a parallel increase in PAI-1 and p53 proteins (Figure 5A). Passive CS exposure for 20 weeks of mice deficient in p53 expression failed to induce PAI-1. Furthermore, ATII cells obtained from mice lacking expression of p53 or PAI-1 were protected from passive CS-induced apoptosis. We next tested whether the p53 binding interaction with PAI-1 mRNA is similarly altered after 20 weeks of passive CS exposure. ATII cells isolated from WT mice exposed to passive CS showed increased PAI-1 mRNA association with p53 protein compared with cells from mice exposed to room air (Figure 5B). In contrast, ATII cells obtained from p53- or PAI-1–deficient mice showed very little p53 interaction with PAI-1 mRNA. To further confirm that p53-induced PAI-1 facilitates apoptosis, lung sections were tested for apoptosis by TUNEL staining and further confirmed by IHC for active caspase-3. As shown in Figure 5Ci (TUNEL) and 5Cii (active caspase-3), mice deficient in expression of p53 or PAI-1 showed resistance to passive CS exposure–induced airway and alveolar epithelial cell apoptosis compared with WT mice. We further analyzed the lung sections for Bak, Bax, and Bcl-XL and found that, unlike in WT mice, neither expression of proapoptotic Bak (Figure 5Ciii) nor Bax (Figure 5Civ) was induced nor was the antiapoptotic Bcl-XL (Figure 5Cv) protein inhibited after passive CS exposure in p53- and PAI-1–deficient mice. Increased active caspase-8 and -10 expressions in lung tissues of mice exposed to passive CS (data not shown) suggest that intrinsic and extrinsic apoptotic pathways synergistically contribute to epithelial lung injury. These findings underscore the importance of newly recognized mechanisms that control ATII cell viability during lung injury.
Figure 5.
p53-mediated induction of PAI-1 expression contributes to CS-induced ATII cell apoptosis in WT mouse lungs. (A) BAL fluids and lysates from ATII cells isolated from WT, p53-deficient, and PAI-1–deficient mice (n = 5 per group) exposed to ambient air or passive CS (PCS) for 20 weeks as described in Figure 4 were analyzed for PAI-1 expression and caspase-3 activation by Western blotting using anti–PAI-1, cleaved, and total caspase-3 antibodies. These membranes containing ATII cell lysates were later stripped and analyzed for β-actin to assess equal loading. The fold change (PCS exposure versus air) in the expression is presented as a bar graph. In the case of ATII cell lysates, the densities of individual PAI-1 and p53 bands were normalized to the corresponding densities of β-actin loading controls. (B) p53 protein immunoprecipitated from the lysates of ATII cells isolated from mouse lungs as described in Figure 5A using anti-p53 antibody. PAI-1 mRNA associated with p53-immune complexes were amplified by RT-PCR in the presence of 32P-labeled dCTP and separated by urea/PAGE, dried, and autoradiographed. (C) Lung sections of WT and p53- and PAI-1–deficient mice as described in Figure 5A were subjected to TUNEL staining (Ci) and immunohistochemical analyses using antiactive caspase-3 (Cii), anti-Bak (Ciii), anti-Bax (Civ) and anti–Bcl-XL (Cv) antibodies to assess changes in apoptosis in situ. Experiments were repeated at least three times, and representative results are illustrated.
We next isolated ATII cells from the lungs of WT mice exposed to passive CS alone or to CS plus CSP or CP, and the lysates were tested for changes in p53 and PAI-1 expression. We found that WT mice exposed to passive CS had elevated levels of p53 and PAI-1 and that these changes were associated with parallel activation of caspase-3 (Figure 6A). Mice exposed to CSP showed minimal p53 and PAI-1 expression. These mice also showed minimal antigens for active caspase-3, demonstrating protection against passive CS–induced ATII cell apoptosis. However, cells obtained from mice exposed to passive CS alone or passive CS plus CP showed augmented p53 and PAI-1 expression with elevated caspase-3 activation. To test if CSP treatment inhibits passive CS exposure–induced p53 interaction with the endogenous PAI-1 mRNA in ATII cells in vivo, we immunoprecipitated p53 and analyzed the associated PAI-1 mRNA. Our data show that passive CS exposure induced p53–PAI-1 mRNA interaction and CSP treatment abrogated coprecipitation of PAI-1 mRNA with the p53 of ATII cells isolated from WT mice (Figure 6B).
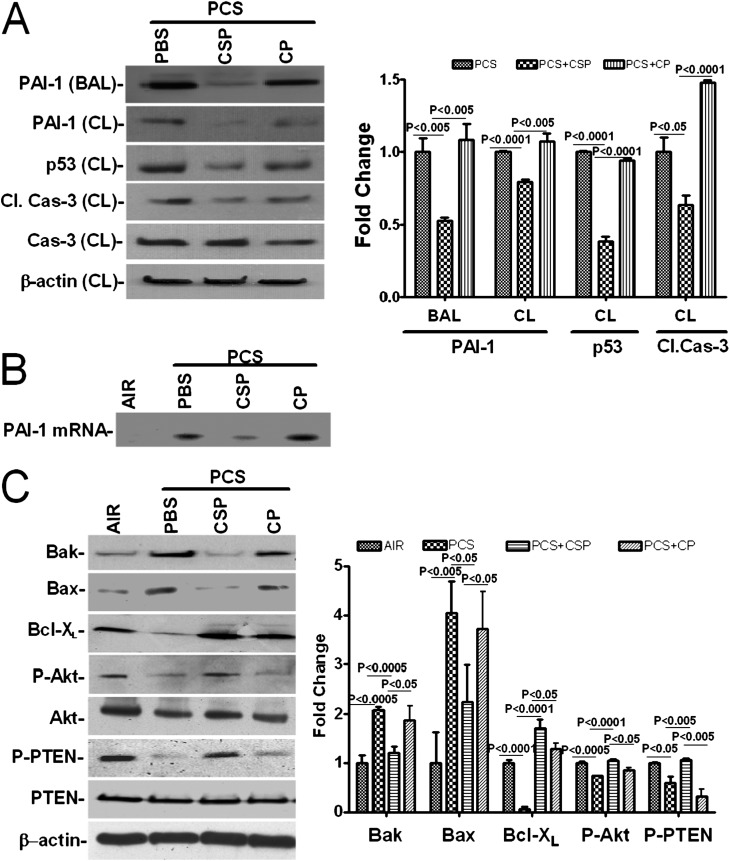
Figure 6.
Inhibition of p53-induced PAI-1 expression by CSP protects against passive CS–induced ATII cell apoptosis in WT mice. (A) BAL fluid and lysates from ATII cells isolated from WT mice (n = 5 per group) exposed to passive CS for 20 weeks as described in Figure 5 in the presence of vehicle or CSP or CP were analyzed for p53 and PAI-1 expression, active/total caspase-3, and β-actin by Western blotting. Densitometric values of individual bands in BAL fluid are presented as fold changes compared with vehicle in the bar graph. In the case of CL, the densities of individual bands were calculated after normalization with corresponding β-actin controls. (B) ATII cells isolated from the lungs of WT mice as described in A were subjected to chemical cross-linking. The immobilized p53–PAI-1 mRNA complexes were immunoprecipitated using anti-p53 antibody, and associated PAI-1 mRNA was amplified by RT-PCR in the presence of 32P-labeled dCTP. (C) Lysates from ATII cells isolated from WT mice as described in Figure 5 were immunoblotted for Bak, Bax, Bcl-XL, phosphorylated Akt (P-Akt), total Akt, phosphorylated PTEN (P-PTEN), total PTEN, and β-actin. The bar graph represents the densitometric values of individual bands normalized against β-actin loading controls and expressed as fold changes. Experiments were repeated at least three times, and representative results are illustrated.
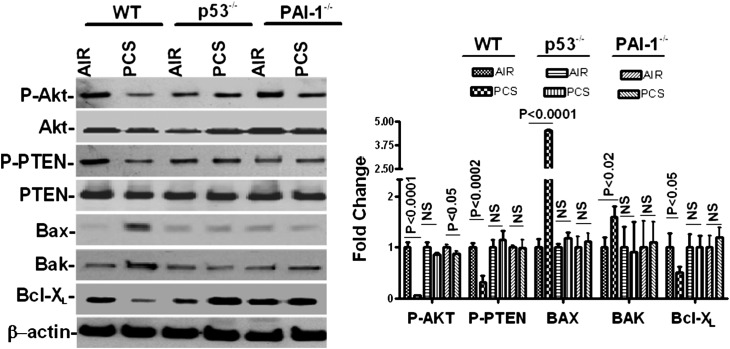
Further analysis of ATII cell lysates for Bax, Bak, and Bcl-XL proteins indicated that CSP treatment inhibited passive CS–induced proapoptotic proteins, such as Bak and Bax, while inducing antiapoptotic protein Bcl-XL expression (Figure 6C). This was consistent with the results of IHC analysis confirming that CSP interferes with the intrinsic pathway. Western blotting for phosphorylated Akt and phosphorylated PTEN showed that passive CS inhibited Akt phosphorylation through upstream phosphatase PTEN and that CSP treatment restored Akt phosphorylation. This indicates the involvement of the Akt survival pathway. To confirm if p53-mediated induction of PAI-1 contributes to ATII cell apoptosis, we analyzed lysates from ATII cells isolated from WT and p53- and PAI-1–deficient mice exposed to passive CS for 20 weeks for phosphorylation of Akt and PTEN and expression of Bak, Bax, and Bcl-XL by Western blotting. The responses were compared with control mice exposed to air. Unlike WT mice, passive CS exposure failed to suppress Akt and PTEN phosphorylation and Bcl-XL expression in p53-deficient mice (Figure 7). However, a slight but significant (P = 0.047) inhibition in Akt phosphorylation was observed in ATII cells of PAI-1–deficient mice exposed to passive CS versus those exposed to ambient air, which could account for the PAI-1–independent responses. p53- and PAI-1–deficient mice also resisted the induction of proapoptotic proteins Bax and Bak expression strongly supporting involvement of p53-induced PAI-1 in ATII cell apoptosis.
Figure 7.
Regulation of ATII cell apoptosis by p53-induced PAI-1 through PTEN-mediated inhibition of Akt phosphorylation during passive CS–induced lung injury. ATII cells isolated from WT, p53-deficient, and PAI-1–deficient mice exposed to ambient air or passive CS for 20 weeks. The cell lysates were tested for phosphorylated Akt (P-Akt), total Akt, phosphorylated P-PTEN, total PTEN, Bax, Bak, Bcl-XL, and β-actin by Western blotting. The bar graph represents the densitometric values of individual bands normalized against β-actin loading controls from three experiments and expressed as fold changes. NS = differences were not statistically significant.
Discussion
CS exposure is the principal contributory factor associated with development of emphysema and an important phenotype of COPD. There is no effective treatment that reverses CS-induced emphysema and/or COPD. There is little detailed information regarding the molecular mechanism contributing to the development of COPD. Multiple studies have demonstrated that airway and alveolar epithelial cell apoptosis and defective alveolar fibrinolysis due to a disproportionate increase in PAI-1 expression are often associated with lung injury. CS contains many toxins that cause DNA damage, and cells with DNA damaged beyond repair undergo apoptosis to protect their genomic integrity.
We demonstrate that p53 and PAI-1 expression, as well as apoptosis by lung epithelial cells and mouse lung epithelium, are induced by CS exposure. Unlike increased PAI-1 gene synthesis by p53 in human osteosarcoma cells (19), CS induces PAI-1 expression through mRNA stabilization at the post-transcriptional level without increasing PAI-1 mRNA synthesis or translation. These are consistent with our recent findings where reintroduction of p53 in p53−/− cells neither increase PAI-1 mRNA synthesis nor mRNA translation (10), indicating that p53-mediated PAI-1 mRNA stabilization is the predominant mechanism of induction of PAI-1 expression. CS exposure increases p53 interactions with PAI-1 mRNA and overexpression of the p53 binding 70 nt PAI-1 mRNA 3′UTR sequence (1) inhibits p53 binding to endogenous PAI-1 mRNA. Furthermore, inability of passive CS to stimulate PAI-1 expression in p53-deficient mice and resistance to airway or alveolar epithelial cell apoptosis by mice deficient in expression for p53 or PAI-1 after passive CS injury demonstrate that p53-induced lung epithelial damage is primarily mediated through increased PAI-1 expression. These assumptions are in agreement with our prior report where treatment of Beas2B cells with p53 siRNA suppressed not only PAI-1 expression but also inhibited CSE-apoptosis in Beas2B cells (10). These findings, along with previously published reports by others (11), suggest that inhibiting the p53 interaction with PAI-1 mRNA could prevent PAI-1 induction and lung epithelial damage induced by CS exposure.
CSP inhibits p53 induction caused by CS exposure, thereby preventing p53 from binding to PAI-1 mRNA 3′UTR, leading to less PAI-1 in ATII cells. PAI-1 expression in CSP-treated ATII cells isolated from WT mice after passive CS exposure was comparable to that observed in p53-deficient cells. These cells express much less PAI-1 compared with cells isolated from CP-treated or untreated WT mice exposed to passive CS. The inhibition of passive CS–induced PAI-1 in ATII cells of CSP-treated mice depends on the extent of suppression of p53. PAI-1 expression in ATII cells after CSP treatment parallels that of cells isolated from ambient air–exposed mice but is significantly less than that of ATII cells isolated from CP-treated mice exposed to CS. We found less p53 and endogenous PAI-1 mRNA complexes in cells of mice treated with CSP after CS injury. The reduction in p53 binding with the PAI-1 mRNA 3′UTR was due to suppression of p53 expression. We could not detect emphysema even at the end of 20 weeks of CS exposure despite a concentration of particulate matter (90 mg/m3) higher than those reported in a study of Irish pubs (21). However, other groups (22–24) have reported emphysema with exposure of mice to higher concentrations (90–350 mg/m3) of particulate matter. Based on the literature (25), it is possible that mice exposed to passive CS for 9 months or more could develop discernible emphysema. Because p53-deficient mice develop spontaneous tumor and die, we could not extend the experiments beyond 20 weeks.
Bax binds and inactivates Bcl-2, and the ratio of Bax to Bcl-2 or Bcl-XL determines the viability of cells (26). Increased expression of the proapoptotic proteins Bax in passive CS–exposed lung sections, and inhibition of their expression after CSP, suggests that Bax could contribute to ATII cell apoptosis. Bax, a proapoptotic member of the Bcl-2 cell death family, is involved in p53-induced apoptosis. The Bax gene promoter region contains p53 consensus sequences, and the latter regulates Bax transcription (27, 28). Therefore, p53 could induce ATII cell apoptosis through induction of Bax, and CSP-mediated protection may involve inhibition of p53-mediated Bax gene transcription. However, PAI-1–deficient mice also resisted ATII cell apoptosis, and the responses of p53- and PAI-1–deficient mice to passive CS were very similar. Therefore, it is unlikely that p53-induced Bax plays a significant role in passive CS–induced ATII cell apoptosis. In that case, alternative intermediaries, such as increased Bak or suppression of Bcl-2, could contribute to the process. Overexpression of Bcl-2 can block p53-dependent apoptosis, and the level of Bcl-2 activity in the cell determines whether the cell will undergo apoptosis or growth arrest (26, 29). CSP induces Bcl-XL in WT mice exposed to passive CS, suggesting its involvement in protecting against ATII cell apoptosis. Because Bcl-XL is one of the five isoforms of the Bcl-X gene predominantly expressed in the lung and is the only isoform that is detectable in respiratory epithelium (30), there is a strong possibility that CSP facilitates protection through induction of Bcl-XL protein. The lack of Bak and Bcl-XL induction after exposure to CS by p53- and PAI-1–deficient mice suggests that they share a common contributory mechanism. Furthermore, CSP restores cell survival probably by suppressing proapoptotic factors and stabilization of antiapoptotic factors by PI3K-dependent Akt phosphorylation to maintain mitochondrial integrity, and the process involves inactivation of phosphatase and PTEN through inhibitory phosphorylation. PTEN is a major negative regulator of PI3K/Akt signaling pathway (31–36) and regulates p53 protein levels and activity, indicating its involvement in p53-mediated downstream changes in PAI-1 and lung epithelial cell viability during passive CS–induced injury. Treatment of ATII cells with recombinant PAI-1 inhibited Akt and PTEN phosphorylation in a dose-dependent manner (data not shown). This strongly supports the involvement of p53 and/or PAI-1–mediated changes in Akt/PTEN signaling in the regulation of ATII cell viability during passive CS–induced lung injury. This is consistent with increased Akt phosphorylation and resistance to apoptosis by PAI-1–deficient endothelial cells due to inactivation of PTEN and reversal of prosurvival effects after exposure of these cells to recombinant PAI-1 (37). Our findings support a new paradigm because passive CS is unable to induce apoptosis in p53- or PAI-1–deficient mice. The data strongly support the novel concept that PAI-1 is responsible for p53-mediated lung epithelial cell apoptosis induced by passive CS.
Supplementary Material
Footnotes
This work was supported in part by grant FAMRI-ID-082380 from Flight Attendant Medical Research Institute Clinical Innovator Award and R21-HL093547 from the National Heart, Lung and Blood Institute.
Originally Published in Press as DOI: 10.1165/rcmb.2011-0390OC on May 17, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.MacNee W, Rahman I. Oxidants and antioxidants as therapeutic targets in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:S58–S65 [DOI] [PubMed] [Google Scholar]
- 2.MacNee W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:50–60 [DOI] [PubMed] [Google Scholar]
- 3.Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, Black S, Shinefield H, Fukuda K. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000;342:232–239 [DOI] [PubMed] [Google Scholar]
- 4.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the united States. JAMA 2004;292:1333–1340 [DOI] [PubMed] [Google Scholar]
- 5.Jeffery PK. Structural and inflammatory changes in COPD: a comparison with asthma. Thorax 1998;53:129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juhan-Vague I, Moerman B, De Cock F, Aillaud MF, Collen D. Plasma levels of a specific inhibitor of tissue-type plasminogen activator (and urokinase) in normal and pathological conditions. Thromb Res 1984;33:523–530 [DOI] [PubMed] [Google Scholar]
- 7.Kruithof EK, Gudinchet A, Bachmann F. Plasminogen activator inhibitor 1 and plasminogen activator inhibitor 2 in various disease states. Thromb Haemost 1988;59:7–12 [PubMed] [Google Scholar]
- 8.Okudela K, Ito T, Mitsui H, Hayashi H, Udaka N, Kanisawa M, Kitamura H. The role of p53 in bleomycin-induced DNA damage in the lung: a comparative study with the small intestine. Am J Pathol 1999;155:1341–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson AJ, Gray RS, Moore NR, Booth NA. The effects of chronic smoking on the fibrinolytic potential of plasma and platelets. Br J Haematol 1997;97:208–213 [DOI] [PubMed] [Google Scholar]
- 10.Shetty S, Shetty P, Idell S, Velusamy T, Bhandary YP, Shetty RS. Regulation of plasminogen activator inhibitor-1 expression by tumor suppressor protein p53. J Biol Chem 2008;283:19570–19580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol 2006;8:877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shetty S, Gyetko MR, Mazar AP. Induction of p53 by urokinase in lung epithelial cells. J Biol Chem 2005;280:28133–28141 [DOI] [PubMed] [Google Scholar]
- 13.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol 1996;14:309–315 [DOI] [PubMed] [Google Scholar]
- 14.Carp H, Janoff A. Possible mechanisms of emphysema in smokers: in vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis 1978;118:617–621 [DOI] [PubMed] [Google Scholar]
- 15.Shetty S, Velusamy T, Idell S, Tang H, Shetty PK. Regulation of urokinase receptor expression by protein tyrosine phosphatases. Am J Physiol Lung Cell Mol Physiol 2007;292:L414–L421 [DOI] [PubMed] [Google Scholar]
- 16.Shetty S, Bhandary YP, Shetty SK, Velusamy T, Shetty P, Bdeir K, Gyetko MR, Cines DB, Idell S, Neuenschwander PF, et al. Induction of tissue factor by urokinase in lung epithelial cells and in the lungs. Am J Respir Crit Care Med 2010;181:1355–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhandary YP, Velusamy T, Shetty P, Shetty RS, Idell S, Cines DB, Jain D, Bdeir K, Abraham E, Tsuruta Y, et al. Post-transcriptional regulation of urokinase-type plasminogen activator receptor expression in lipopolysaccharide-induced acute lung injury. Am J Respir Crit Care Med 2009;179:288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shetty S, Bdeir K, Cines DB, Idell S. Induction of plasminogen activator inhibitor-1 by urokinase in lung epithelial cells. J Biol Chem 2003;278:18124–18131 [DOI] [PubMed] [Google Scholar]
- 19.Kunz C, Pebler S, Otte J. von der Ahe D. Differential regulation of plasminogen activator and inhibitor gene transcription by the tumor suppressor p53. Nucleic Acids Res 1995;23:3710–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhandary YP, Shetty SK, Marudamuthu AS, Gyetko MR, Idell S, Gharaee-Kermani M, Shetty RS, Starcher BC, Shetty S. Regulation of alveolar epithelial cell apoptosis and pulmonary fibrosis by coordinate expression of components of the fibrinolytic system. Am J Physiol Lung Cell Mol Physiol 2012;302:L463–L473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulcahy M, Byrne MA, Ruprecht A. How does the Irish smoking ban measure up? A before and after study of particle concentrations in Irish pubs. Proceedings: Indore Air 2005;15:1659–1662 [Google Scholar]
- 22.Foronjy RF, Mercer BA, Maxfield MW, Powell CA, Armiento JD. Structural emphysema does not correlate with lung compliance: lesson from the mouse smoking model. Exp Lung Res 2005;31:547–562 [DOI] [PubMed] [Google Scholar]
- 23.Morrow SAM, Lauer T, Collaco JM, Yee M, Reilly MO, Mitzner W, Neptune E, Wise R, Biswal S. Neonatal hyperoxia contributes additively to cigarette smoke–induced chronic obstructive pulmonary disease changes in adult mice. Am J Respir Cell Mol Biol 2011;45:610–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujihara M, Nagai N, Sussan TE, Biswal S, Handa JT. Chronic cigarette smoke causes oxidative damage and apoptosis to retinal pigmented epithelial cells in mice. PLoS ONE 2008;3:e3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simet SM, Sisson JH, Pavlik JA, Devasure JM, Boyer C, Liu X, Kawasaki S, Sharp JG, Rennard SI, Wyatt TA. Long term cigarette smoke exposure in a mouse model of ciliated epithelial cell function. Am J Respir Cell Mol Biol 2010;43:635–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu A, Haldar S. The relationship between bcI2, bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod 1998;4:1099–1109 [DOI] [PubMed] [Google Scholar]
- 27.Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC. Tumor suppressor P53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 1994;9:1799–1805 [PubMed] [Google Scholar]
- 28.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995;80:293–299 [DOI] [PubMed] [Google Scholar]
- 29.Chiou SK, Rao L, White E. Bcl-2 blocks p53-dependent apoptosis. Mol Cell Biol 1994;14:2556–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staversky RJ, Vitiello PF, Yee M, Callahan LM, Dean DA, O'Reilly MA. Epithelial ablation of bcl-xl increases sensitivity to oxygen without disrupting lung development. Am J Respir Cell Mol Biol 2010;43:376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/akt pathway. Proc Natl Acad Sci USA 1999;96:4240–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan X, Helman LJ. Levels of PTEN protein modulate akt phosphorylation on serine 473, but not on threonine 308, in IGF-II-overexpressing rhabdomyosarcomas cells. Oncogene 2003;22:8205–8211 [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/akt pathway. Proc Natl Acad Sci USA 1998;95:15587–15591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase akt pathway in human cancer. Nat Rev Cancer 2002;2:489–501 [DOI] [PubMed] [Google Scholar]
- 35.Georgescu MM. PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer 2010;1:1170–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, et al. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 2003;3:117–130 [DOI] [PubMed] [Google Scholar]
- 37.Balsara RD, Castellino FJ, Ploplis VA. A novel function of plasminogen activator inhibitor-1 in modulation of the AKT pathway in wild-type and plasminogen activator inhibitor-1-deficient endothelial cells. J Biol Chem 2006;281:22527–22536 [DOI] [PubMed] [Google Scholar]
- 38.Shetty S. Regulation of urokinase receptor mRNA stability by hnRNP C in lung epithelial cells. Mol Cell Biochem 2005;272:107–118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.