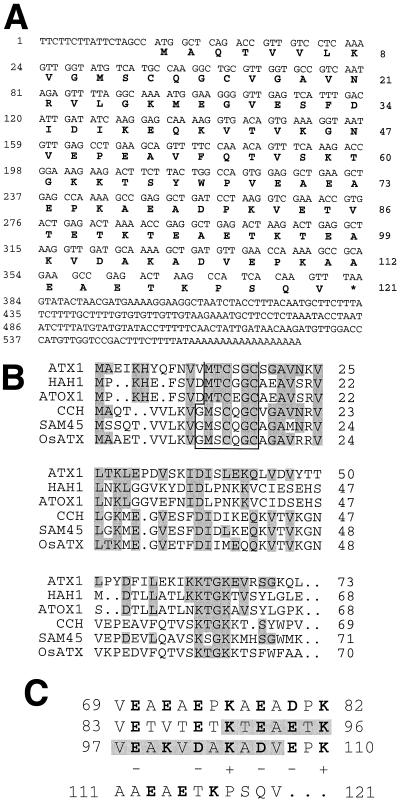
Figure 1.
CCH sequence analysis. A, Nucleotide and predicted amino acid sequences for the CCH cDNA. B, Alignment of yeast ATX1 with the predicted amino acid sequence of HAH1 (human), ATOX1 (mouse; accession no. AF004591), and the 69 N-terminal amino acids of CCH (Arabidopsis), SAM45 (soybean), and rice ATX1 (OsATX1; accession no. DBEST 70798). Conserved residues in the alignment are shaded. The putative metal-binding domain is boxed. C, Amino acid sequence features of the C-terminal region of CCH. Charged residues are shown in boldface and their charges are indicated below them. Secondary-structure prediction suggested that an α-helix could be formed in the shaded region.