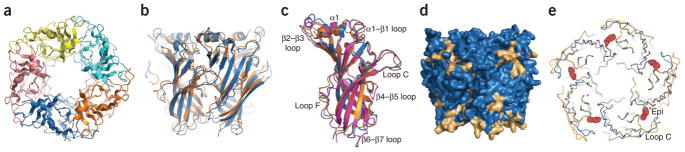
Figure 2.
Overall structures of the α7–AChBP chimera and comparison to related structures. (a) Top view of the α7–AChBP chimera pentamer along the five-fold axis of symmetry; each subunit is shown in a different color. (b) Structure superposition between the α7–AChBP chimera (blue) and AChBP (orange) pentamers viewed from the side that is normal to the five-fold axis. (c) Structure superposition of subunits from the α7–AChBP chimera (blue), α1 extracellular domain (magenta) and AChBP (orange); loops showing substantial differences are labeled. (d) Surface representation showing α7 residues (blue) and AChBP residues area (beige) on the α7–AChBP chimera. (e) Backbone superposition between the Apo (gold) and Epi (blue) structures viewed down the five-fold axis. The epibatidine molecule (Epi) is shown by the Fo − Fc electron density contoured at the 3.0-σ level.