Table 1. Data-collection and refinement statistics.
Values in parentheses are for the highest resolution shell.
| D1 data set | D2 data set | D1D2 data set | D3 data set | |
|---|---|---|---|---|
| Data collection | ||||
| X-ray source | APS 22-ID | APS 22-ID | APS 22-ID | |
| Wavelength (Å) | 1.9 | 1.9 | 0.9724 | |
| Crystal-to-detector distance (mm) | 125 | 125 | 230 | |
| Exposure time (s) | 3 | 2 | 1 | |
| Space group | P42 | P42 | P42 | P42 |
| Unit-cell parameters (Å) | a = b = 53.54, c = 41.25 | a = b = 53.52, c = 41.24 | a = b = 53.54, c = 41.25 | a = b = 53.03, c = 40.97 |
| Resolution range (Å) | 50.00–2.30 (2.38–2.30) | 50.00–2.30 (2.38–2.30) | 50.00–2.30 (2.38–2.30) | 50.00–1.85 (1.92–1.85) |
| Completeness (%) | 95.9 (59.3) | 99.8 (98.5) | 99.9 (99.2) | 99.9 (99.8) |
| Redundancy | 12.0 (3.9) | 13.5 (9.7) | 25 (13.4) | 13.3 (12.5) |
| R merge † (%) | 4.1 (32.3) | 4.1 (21.3) | 4.5 (25.4) | 5.1 (23.3) |
| 〈I/σ(I)〉 | 72.4 (3.1) | 94.1 (14.5) | 124.7 (17.38) | 60.66 (12.96) |
| Mosaicity (°) | 0.48–0.65 | 0.50–0.65 | 0.56 | |
| Unique reflections | 5347 | 5325 | 5325 | 9859 |
| Rmeas ‡ (%) | 4.4 (36.2) | 4.2 (22.2) | 4.8 (28.4) | |
| R p.i.m. § (%) | 1.2 (16.1) | 1.1 (6.7) | 0.9 (7.5) | |
| SHELXD CCall/CCweak | 40.8/11.2 | 29.75/13.49 | 47.93/29.15 | |
| RTSI (see text) | 43.33 | 37.63 | 68.00 | |
| Traceable map | No | No | Yes | |
| Refinement | ||||
| Resolution limits (Å) | 37.86–2.30 | 37.50–1.85 | ||
| Reflections used | 5325 | 9761 | ||
| No. of protein atoms refined | 740 | 748 | ||
| No. of water molecules | 32 | 76 | ||
| R work/R free ¶ (%) | 23.2/26.8 | 17.90/21.40 | ||
| R.m.s.d. bond lengths (Å) | 0.009 | 0.008 | ||
| R.m.s.d. bond angles (°) | 1.300 | 0.998 | ||
| Mean B value (Å2) | 50.0 | 28.7 | ||
| MolProbity all-atom clash score | 20.42 | 12.35 | ||
| Ramachandran favored (%) | 87/88 | 88/89 | ||
| PDB entry | 3o3k †† | 3ov8 | ||
R
merge = 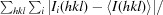
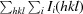 , where I(hkl) is the observed intensity of reflections.
, where I(hkl) is the observed intensity of reflections.
R meas is the redundancy-independent merging R factor of Diederichs & Karplus (1997 ▶).
R p.i.m. is the precision-indicating merging R factor of Weiss & Hilgenfeld (1997 ▶).
R
work = 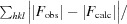
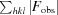 , where F
calc and F
obs are the calculated and observed structure-factor amplitudes, respectively. R
free is the same as R
work but calculated using an ∼5% test set of reflections that were excluded from refinement.
, where F
calc and F
obs are the calculated and observed structure-factor amplitudes, respectively. R
free is the same as R
work but calculated using an ∼5% test set of reflections that were excluded from refinement.
The accompanying 2.3 Å resolution S-SAD data set has been deposited in the PDB.
