Abstract
Intervertebral disc (IVD) pressure measurement is an appropriate method for characterizing spinal loading conditions. However, there is no human or animal model that provides sufficient IVD pressure data. The aim of our study was to establish physiological pressure values in the rabbit lumbar spine and to determine whether temporary external disc compression and distraction were associated with pressure changes. Measurements were done using a microstructure-based fibreoptic sensor. Data were collected in five control rabbits (N, measurement lying prone at segment L3/4 at day 28), five rabbits with 28 days of axial compression (C, measurement at day 28) and three rabbits with 28 days of axial compression and following 28 days of axial distraction (D, measurement at day 56). Disc compression and distraction was verified by disc height in lateral radiographs. The controls (N) showed a level-related range between 0.25 MPa–0.45 MPa. The IVD pressure was highest at level L3/4 (0.42 MPa; range 0.38–0.45) with a decrease in both cranial and caudal adjacent segments. The result for C was a significant decrease in IVD pressure (0.31 MPa) when compared with controls (P=0.009). D showed slightly higher median IVD pressure (0.32 MPa) compared to C, but significantly lower levels when compared with N (P=0.037). Our results indicate a high range of physiological IVD pressure at different levels of the lumbar rabbit spine. Temporary disc compression reduces pressure when compared with controls. These data support the hypothesis that temporary external compression leads to moderate disc degeneration as a result of degradation of water-binding disc matrix or affected active pumping mechanisms of nutrients into the disc. A stabilization of IVD pressure in discs treated with temporary distraction was observed.
Keywords: Axial disc distraction, Disc degeneration, Intradiscal pressure, In vivo animal model
Introduction
Intervertebral disc (IVD) pressure is an important parameter to characterize spinal overload in disc degeneration [23]. It has been reported to be one of the few determinants that is directly influenced by axial spinal load [21]. The pioneering scientific work was done by Nachemson et al. in the 1960s and 1970s. Nachemson showed a relationship between different body positions or exercises (unsupported sitting, reclining, forward leaning and lifting weights) and lumbar IVD pressure [14] . Wilke et al. [30] partially confirmed these early in vitro data when they performed in vivo IVD pressure experiments in one healthy volunteer. Physiological IVD pressure was assumed to range between 0.1 MPa–0.24 MPa with the volunteer lying prone throughout the night.
However, IVD pressure measurements are generally not used in the clinical management of disc disease [15, 17, 20–22, 25, 29]. As a result of its invasive nature, there have been mostly in vitro approaches and only one human in vivo study reported a negative correlation between the degree of disc degeneration and IVD pressure [21]. Also, a pressure decline of 25% in 38 human cadaver lumbar discs with mechanical endplate injury has been shown [1].
To explain the mechanism of IVD pressure decline in disc degeneration, it is fundamental to consider disc morphology. The complex structure of the healthy nucleus pulposus consists of only few chondrocyte-derived cells [23]. They are embedded in an extracellular matrix consisting of hydrophilic proteoglycans. This is the reason for the 80% water content and the swelling pressure inside the nucleus pulposus. The hydrostatic pressure is constrained by two anatomic structures: the ligament-like anulus fibrosus around the periphery and the endplates attached to the adjacent vertebral bodies [6]. The nucleus cells and, cells associated by invasion of blood vessels [18], are stimulated by mechanical compression. These cells respond with an upregulation of matrix degrading factors, which results in a reduction in hydrophilic proteoglycans explaining the decreased pressure in disc degeneration [8, 28].
We established an in vivo rabbit model capable of creating disc degeneration. The mechanism is an external application of controlled and far hyper-physiological axial compression to one lumbar spine disc [10, 26]. Recently we reported that axial distraction could be a therapeutic option in degenerative disc disease [12]. The aim of this study was (a) to measure IVD pressure in healthy discs, (b) to compare the results with those of compressed discs (c) to determine the effects of axial distraction in previously compressed discs. We hypothesise that compression loading results in lower disc pressure and axial distraction can re-establish physiological values.
Materials and methods
Animals
Sixteen New Zealand rabbits were used according to a protocol approved by the Institutional Review Board of the Animal Experimentation Committee Karlsruhe, Germany. The weight of the animals ranged from 2.8 kg to 4.2 kg. The age was 0.5 years and skeletal status was mature. All rabbits were randomly assigned to one of three groups:
N Normal control rabbits, 28 days untreated; n=5 (N1–N5)
C Compression group, 28 days of axial compression; n=6 (C1–C5; one exclusion)
D Distraction group, 28 days of compression, followed by 28 days of distraction; n=5 (D1–D3; two exclusions)
The fibreoptic pressure sensor system (Samba Sensors AB, Sweden)
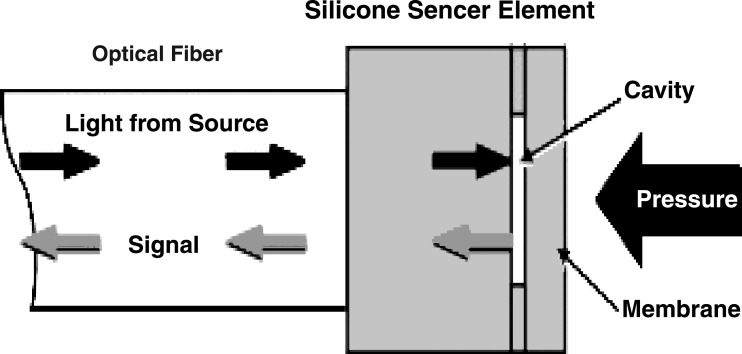
A fibreoptic device was used to determine IVD pressure. The system consisted of a silicon sensor element and an optical fibre to which it was attached. The silicon sensor element was attached to two layers of silicon (Fig. 1). One of these double wavers was formed to a well-defined cavity with photolithographic and wet etching techniques. There was a membrane placed within this Fabry-Perot cavity. A light source, by means of a light emitting diode, was located into the fibre end opposing the sensor. The emitted light signal was conducted through the fibre and then reflected by the sensor. An external pressure application deflected the silicon, and the membrane and depth of the cavity changed. This led to optical interferences during reflection within the sensor. The generated light variations were determined by the photo detector located at the sensor-opposing end of the fiber. The detector signals were preamplified and further processed [7].
Fig. 1.
The samba sensor principle
All data are expressed in bar or MPa. A frequency of 5 Hz was found to be appropriate. To determine the reproducibility of measurements, the in vitro precision error was estimated. For this, ten repeated measurements were performed with different disc punctures. The standard deviation of the measurements was calculated and divided by the mean of measurements. Thus, an in vitro precision error of 0.0669 was obtained.
Surgical procedures and the IVD pressure measurement
Under general anaesthesia, rabbits of group C and group D were placed in prone position. Dorsal to the lumbar spine a custom-made external loading device was attached to the vertebra body L3 and L4 [10]. After the wound was closed, axial stress to the disc was immediately created by a calibrated spring within the loading device so as to produce an external disc compressive force of approximately 200 N. Postoperatively, all animals were allowed free unrestricted weight bearing and activity in cages. All rabbits were monitored daily. In group D, the external compression device was removed after 28 days and replaced by the external distraction device [12]. A distraction load of approximately 120 N was applied. Early in vivo experiments showed that the external compression of 200 N leads to a pressure of 0.9 MPa inside the nucleus, and the external distraction load of 120 N reduces the internal pressure to 0 MPa.
We performed both in vivo and ex vivo measurements. For in vivo measurements, the rabbit underwent a second, more extensive posterior-lateral approach to the spine in general anesthesia. To obtain broad access to the IVD, incision from the iliac crest to the spinous process of L2 was carried out. After exposing, the disc L3/4 was punctured horizontal by a cannula (G 18). Afterwards, the needle was withdrawn, and the sensor was inserted simultaneously without nucleus leakage. The correct position inside the nucleus pulposus was confirmed by fluoroscopy. (Fig. 2a). Due to the anatomy of the rabbit lumbar spine, it was difficult to obtain in vivo IVD pressure data with adequate data reproducibility. This was reinforced by the inconsistent path of the processus transversus in the lumbar spine of rabbits. In some of the animals, the processus transversus was large and positioned directly lateral to the IVD (Fig. 2b–d]. To get ex vivo measurement results, the rabbits were sacrificed after in vivo procedure. Immediately after harvesting the whole thoracic and lumbar spine was placed in situ with the animal lying prone with attached muscle and soft tissue similar to the in vivo experiment. The processi transversi were resected and the same procedure was performed as described above. Pre-experiments in two animals determined no significant pressure difference between in vivo and ex vivo performance. The ex vivo measurement was performed approximately 15 min after animal sacrifice. For data consistency, all measurements included in the statistical analysis were in vitro procedures. Measurement recording time was at least three 3 min to provide sufficient accuracy. The temporal variation was below 0.001 MPa over 3 min in all measurements. All measurements were performed with the loading device removed.
Fig. 2.
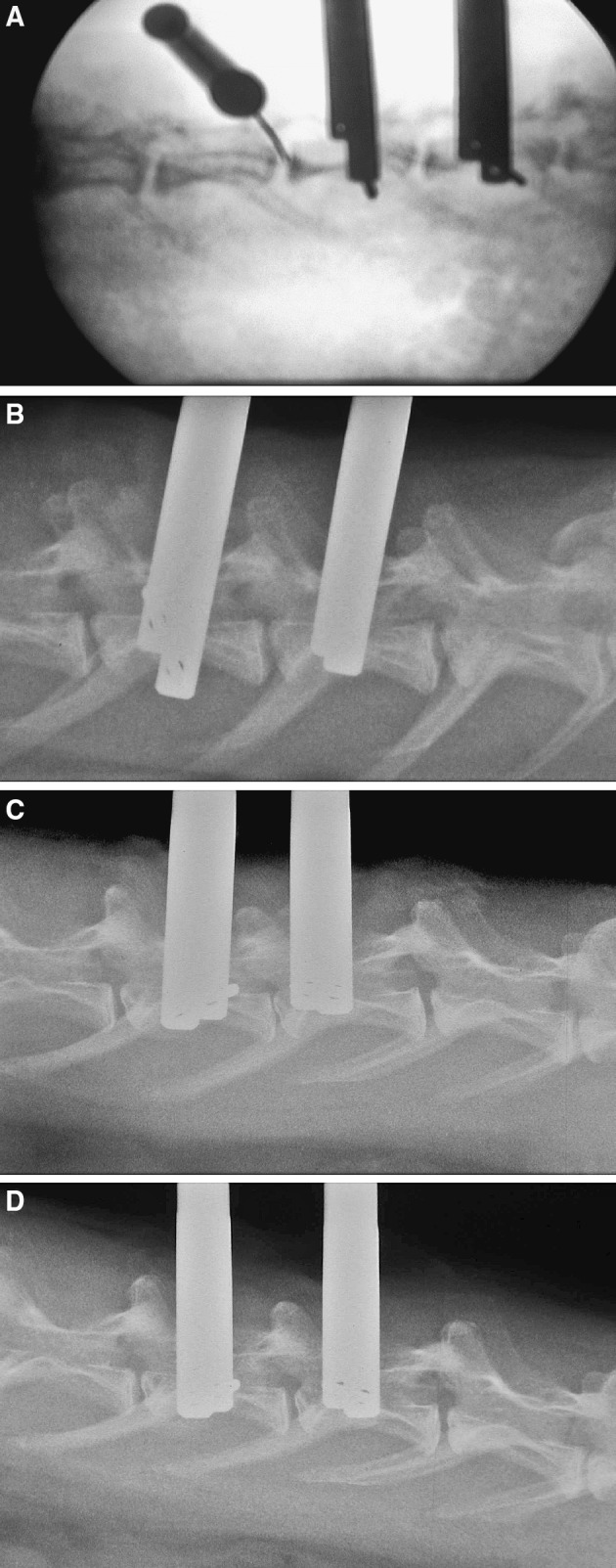
a Radiographic verification of correct sensor placement inside the nucleus pulposus of L2/3 segment adjacent to a loaded disc. b Lateral Radiograph of the lumbar spine before application of load. Note the processus transversus located laterally the intervertebral discs. Median physiological disc height at L3/4 was 2.01 mm. c Lateral radiograph of the lumbar spine after application of temporary disc loading. Note slight endplate reactions at the loaded segment and the significant disc height reduction (1.47 mm, P=0.009 vs N). d Lateral radiograph of the lumbar spine after application of temporary disc loading and axial distraction. Note the disc height re-establishment (2.02 mm, P=0.836 vs N)
To compare the three groups, IVD pressure measurement points were arranged as follows:
Control group (N): after 28 days of no treatment
Compression group (C): day 28 after constant application of external compression
Compression and distraction group (D): day 56 after 28 days of axial compression and 28 days of axial distraction.
Radiology
All harvested specimens were used for radiological examination to determine disc height. Disc degeneration was verified by a decrease in disc height, disc distraction by a re-establishment of normal disc height (Fig. 2b–d). Lateral radiographs were obtained after 28 days (C) or after 28 and 56 days (D). The radiographs were digitized using a flatbed scanner with backlighting and passed to a computer on which images were magnified. Under lateral view the disc height was marked and measured in each segment. Measurement for disc thickness was calculated using X-rays to make two linear and angular measurements of calibration standards. The average disc thickness of each specimen was then calculated.
Statistical analysis
Statistical analysis was performed using the dependent outcome parameters “disc pressure” and “disc height” at the treated segment (L3/4) and adjacent segments (L2/3; L4/5); “group” (N; C; D) was the independent variable. Primary outcome measure was the comparison of outcome parameters. To determine measures of central tendencies and distribution, median and range were calculated. Further non-parametric analysis was done step-wise to analyze whether independent samples come from the same population: the Kruskal–Wallis test with the dependent variable “group” (N, C, D) was performed as global test, respectively, a post-hoc analysis was done with the Mann–Whitney U-test. Because of the small sample size Monte Carlo–simulation–method was performed. This statistical method eliminates the risk of incorrect tests in the case of small sample size and non-normal distribution, because it is not based on assumptions about the distribution of expression values or the equality of variance. Thus, the Monte–Carlo–simulation for the Mann–Whitney U-test is useful in the case of samples with expected cell frequencies smaller than five [19, 31]. A two-tailed P≤0.05 was considered significant. Because of the exploratory design of this study we did all tests without alpha adjustment. Finally, a power analysis was performed to determine the probability that the study with given size would in fact detect as statistically significant a real difference of the given magnitude. Thus, a power of 0.8 was required [UCLA Department of Statistics (2004) Power Calculator, Los Angeles]. Data analysis was performed with SPSS for Windows 12.0 (SPSS Inc., Chicago, USA).
Results
Animals
Sixteen animals were used for this experiment. Of these13 were followed to the end of the study without restriction in movement ability or weight loss (five in N and C, three in D). One excluded animal experienced neurological deficiencies, another had inward aggression with dismemberment of his right lower extremity and one was excluded because of vertebral body fracture with disc injury (D).
Radiological disc height
In controls (N), IVD thickness of L3/4 averaged 2.01 mm in all specimens. A significant decrease in disc thickness after 28 days of external compression (C) was found (1.47 mm, P=0.009). No fusion of the treated segment occurred, however, signs of endplate sclerosis were found (Fig. 2c). In D, disc thickness after 28 days of compression and 28 days of distraction was not significantly changed in controls (2.02 mm, P=0.836). Disc height adjacent to loaded discs did not significantly differ between groups (Table 1).
Table 1.
Intervertebral disc pressure (press, MPa) and radiographic disc height (height, mm) at disc levels L2/3, L3/4 and L4/5
| Number | Pres L2/3 | Pres L3/4 | Pres L4/5 | Height L2/3 | Height L3/4 | Height L4/5 |
|---|---|---|---|---|---|---|
| N1 | 0.35 | 0.39 | 0.38 | 2.1 | 1.98 | 2 |
| N2 | 0.33 | 0.38 | 0.25 | 1.94 | 2.01 | 2.1 |
| N3 | 0.28 | 0.43 | 0.37 | 1.82 | 1.89 | 2 |
| N4 | 0.37 | 0.45 | 0.33 | 2.02 | 2.12 | 2.1 |
| N5 | 0.38 | 0.42 | 0.34 | 2.0 | 2.02 | 2.02 |
| Median | 0.35 | 0.42 | 0.34 | 2.0 | 2.01 | 2.02 |
| C1 | 0.33 | 0.31 | 0.38 | 1.93 | 1.48 | 2.05 |
| C2 | 0.37 | 0.37 | 0.38 | 1.89 | 1.41 | 1.76 |
| C3 | 0.52 | 0.13 | 0.31 | 1.81 | 1.37 | 1.98 |
| C4 | 0.32 | 0.36 | 0.23 | 2.12 | 1.78 | 2.12 |
| C5 | 0.37 | 0.31 | 3.68 | 2.05 | 1.47 | 2.02 |
| Median | 0.37 | 0.31 | 0.37 | 1.93 | 1.47 | 2.02 |
| D1 | 0.43 | 0.25 | 0.40 | 1.84 | 2.23 | 2.01 |
| D2 | 0.31 | 0.32 | 0.30 | 2.04 | 1.88 | 1.79 |
| D3 | 0.49 | 0.35 | 0.23 | 1.83 | 2.02 | 1.78 |
| Median | 0.43 | 0.32 | 0.30 | 1.84 | 2.02 | 1.79 |
Comparison between the control group N (number N1–N5), the compression group (C1–C5) and the distraction group (D1–D3)
Physiological IVD pressure (N)
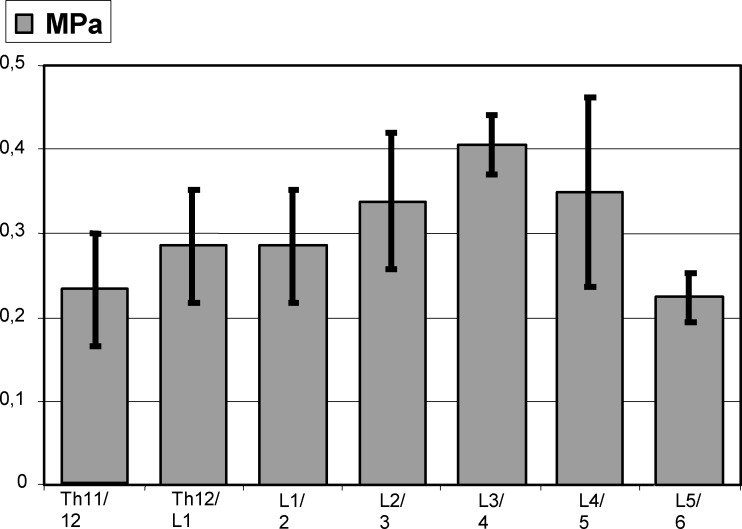
In N, median IVD pressure was 0.36 MPa (n=15) with highest values at L3/4 (0.42 MPa, n=5, 0.38–0.45) and lowest levels at L5/6 (0.22 MPa) (Fig. 3). A distribution with highest pressure at L3/4 and a decrease in adjacent discs was found in controls. The range in N was 0.25–0.45 MPa, compared with a less extensive level-specific range: 0.28–0.38 MPa for L2/3, 0.38–0.45 MPa for L3/4 and 0.25–0.38 MPa for L4/5 (Table 1). The median level-related pressure range was 0.1 MPa for all disc segments.
Fig. 3.
Intervertebral disc pressure measurements (MPa) in healthy control discs (N) with standard deviations, n=5, spine level TH11/12-L5/6
IVD pressure measurements after compression (C) and distraction following compression (D) treatment
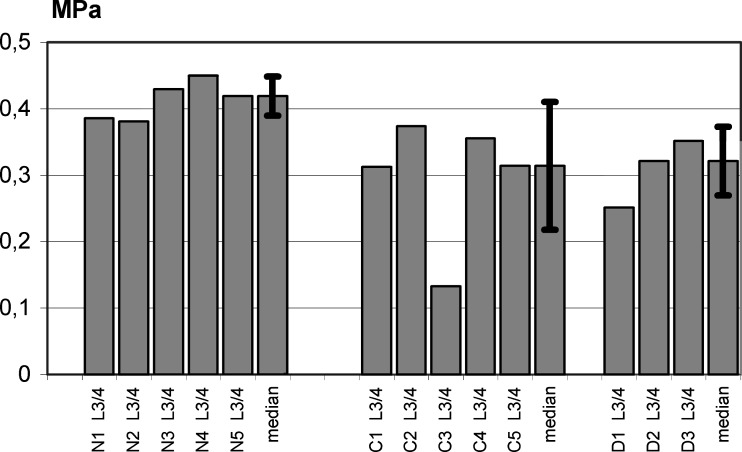
In C, pressure was 0.31 MPa (n=5, 0.13–0.37 MPa) at L3/4 In D, pressure decreased to 0.32 MPa (n=3, 0.25–0.35) at L3/4 (Table 1, Fig. 4). The global statistic test (Kruskal–Wallis variance analysis) for L3/4-values showed a statistically significant change between the control group (N) and the treatment groups (C and D) (n=13, chi-square=8.58, df=2, P=0.004). When comparing C with N, the Mann–Whitney U-test was significant (P=0.009) with sufficient statistical power (0.995). Comparison between C and D demonstrated no statistically significant difference (P=0.999; power 0.037), however, in D significantly lower pressures were found compared with N (P=0.037; power 0.852).
Fig. 4.
Intervertebral disc pressure measurements (MPa) and standard deviation at spine level L3/4: comparison between controls (N1–N5, median), compressed (C1–C5, median) and distracted (D1–D3, median) discs. In both C and D, IVD pressure was significantly reduced compared to controls (C: P=0.009; D: P=0.037) while no significant difference was found between C and D (P=0.852)
Discussion
This study examined IVD pressure in untreated controls, in mechanically compressed discs and in discs treated with axial distraction following disc compression. The results demonstrated a wide range of physiological values at different levels of the rabbit lumbar and lower thoracic spine. A distribution with highest pressure at L3/4 and a decrease towards cranial and caudal segments was found. The lowest IVD pressure in the rabbit spine was measured at L5/6, followed by TH11/12 and Th12/L1. This can be due to higher mechanical stress at the transition zone from lumbar spine to Os sacrum and thoracic spine respectively. Although the high range of physiological IVD pressure was not expected, the interindividual range of pressure at corresponding levels of the non-treated rabbit spine was far less extensive. Most reported studies on IVD pressure measurements examined one single disc [2, 14, 15, 20–22, 30] or the adjacent discs to fusion [4, 5, 16, 25, 29]. We conclude that it is important to compare only corresponding levels of the spine with respect to intradiscal pressure measurements.
There are few studies that have investigated the association of IVD pressure and degeneration of the disc. Sato et al. [21] performed disc pressure measurements in subjects with or without ongoing back pain after assessing the degree of disc degeneration by MRI. The intradiscal pressure in subjects with significant disc degeneration was significantly reduced compared with that of healthy discs.
Results of our compression group determined a significant decrease in IVD pressure at L3/4. All animals of this group showed lower intradiscal pressure when compared with N. Additionally, load application resulted in lower disc height in lateral radiograph, which verified disc compression and approximated the degree of disc degeneration. As reported earlier, morphology showed degenerative changes after 28 days of axial compression [10]. We believe that the IVD pressure decrease in compressed discs is consistent with earlier findings and support the concept of moderate disc degeneration.
The time disc cells need to reorganize after tissue injury remains unknown. It is possible that the process of restructuring is not fully completed after 28 days of compression treatment. This is supported by reports from Hutton et al. [8] showing first visible changes after compression treatment on a gene expression level hours to days after initiating load. In contrast, on a biochemical basis visible changes were found after months. Accordingly, it seems possible that in our model a prolonged time of compression leads to more severe disc degeneration with further decreased pressure. This might reflect the nature of chronic disc disease in humans as a result of a long-term chronic injury of disc tissue.
Disc degeneration in animal models is still a matter of debate [1, 8–13]. Most studies with mechanically induced disc degeneration demonstrated a correlation between compression force and disc degeneration [1, 9, 10, 13]. There may be a threshold at which forces stimulate tissue degeneration due to a complex pathomechanism. This mechanism may be initiated through changes in cellular and extracellular shape caused by mechanical stress or an adverse biochemical environment produced by water loss. We noted that an application of 200 N increases intradiscal pressure to 0.9 MPa from a baseline of 0.3 MPa. The load was applied in five equal steps for 60 s. Pressure increase was approximately 0.1 MPa per step and lasted for 60 s. However, after application of the total load, the pressure of 0.9 MPa was slightly decreased with time exceeding several minutes. This is most likely due to a fluid expression from the nucleus pulposus and the establishment of a new equilibrium. We conclude that the external mechanical application of 200 N is sufficient to initiate protein degradation or to affect water nutrition.
Comparable animal models applied an external load between 0.15 MPa and 1.3 MPa [10, 12, 15], which may be below the postulated threshold for tissue degradation. The frequency and amount of load application seems to play a crucial role in disc metabolism. Walsh and Lotz [27] demonstrated that dynamic mechanical forces were important regulators in vivo of disc cellularity and matrix synthesis. No significant changes in morphology, proteoglycan content or cell death were found after loading at 0.9 MPa, 0.1 Hz. Loading at lower frequency and/or higher stress increased proteoglycan content, matrix gene expression and cell death. The biochemical details of these mechanisms still remain unknown and further studies are needed.
The result for the distraction group showed a pressure decrease when compared with controls. No significant change in pressure was observed between groups C and D. The lateral radiographs showed an increase in disc height at this disc to 2.02 mm, equal to controls (2.01 mm). Posterior axial disc distraction after external disc compression leads to a restitution of disc height and does lead to slightly increased pressure values. Knowledge of disc pressures after disc compression and following physiological loading conditions is sure. There are a number of studies that investigated the influence of hydrostatic and osmotic pressure on disc tissue. Chen et al. showed that under hypo-osmotic (255 mOsm) conditions in vitro gene expression of aggrecan and collagen type II was upregulated in the transition zone of the disc [3]. In contrast, expression within the nucleus pulposus was not significantly changed. By performing an axial posterior distraction, we expect an increase of free water inside the disc tissue with decreased osmolarity especially inside the nucleus pulposus. This may contribute to the fact that IVD pressure in our study was not increased, but the overall disc reorganization is stimulated [11, 12].
Combining this data with previously published studies, a final evaluation of posterior axial distraction seems to be premature with respect to disc recovery. However, data of cellular responses in animal models demonstrated an increase in apoptotic cells by temporary disc compression. After temporary axial disc distraction, the number of apoptotic cells returned to physiological levels [11]. Additionally, axial distraction stopped or even partly reversed compression-induced degeneration on a histological basis [12]. A human study reported the use of a novel dynamic neutralization non-fusion system, which is based on a similar distraction mechanism. Patients with degenerative disc disease had significantly improved clinical symptoms [24], although it remains unclear whether disc status can be improved by implanting this device. Further studies, especially more data on the detailed molecular processes of disc reorganization and disc nutrition after temporary posterior disc distraction, are needed to clarify this issue.
Conclusions
Intervertebral disc pressure measurement with a fibreoptic device is an accurate method of evaluating disc status without injuring the disc significantly. The physiological range of pressure in the lumbar spine was high with a maximum at segment L3/4 and a decrease in both cranial and caudal segments. Axial compression treatment leads to a decrease in IVD pressure, consistent with moderate disc degeneration. No further decrease is detected by dynamic axial distraction.
Acknowledgements
This work was supported by the Department of Orthopaedic Surgery at the University of Heidelberg. The authors have received nothing of value from a private or commercial entity for the data described in this study.
References
- 1.Adams MA, Freeman B, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;13:1625–1636. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Castagnera L, Grenier N, Lavignolle B, Greselle JF, Senegas J, Caille JM. Study of correlation between intradiscal pressure and magnetic resonance imaging data in evaluation of disc degeneration. Therapeutic issue with percutaneus nucleotomy. Spine. 1991;16(3):348–352. doi: 10.1097/00007632-199103000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Baer AE, Paik PY, Yan W, Setton LA (2002) Matrix protein gene expression in intervertebral disc cells subjected to altered osmolarity. Biochem Biophys Res Commun 10;293(3):932–938 [DOI] [PubMed]
- 4.Cunningham BW, Kotani Y, McNulty PS, Cappuccino A, McAfee PC. The effect of spinal destabilisation and instrumentation on lumbar intradiscal pressure. An in vitro biomechanical analysis. Spine. 1997;22:2655–2663. doi: 10.1097/00007632-199711150-00014. [DOI] [PubMed] [Google Scholar]
- 5.Eck JC, Humphreys SC, Lim TH, Jeong ST, Kim JG, Hodges SD, An HS. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine. 2002;27(22):2431–2434. doi: 10.1097/00007632-200211150-00003. [DOI] [PubMed] [Google Scholar]
- 6.Freemont AJ, Watkins A, Le Maitre C, Jeziorska M, Hoyland JA. Current understanding of cellular and molecular events in intervertebral disc degeneration: implications for therapy. J Pathol. 2002;196:374–379. doi: 10.1002/path.1050. [DOI] [PubMed] [Google Scholar]
- 7.Höjer S, Krantz M, Ekström L, Kaigle A, Holm S. A microstructure based fiberoptic pressure sensor for measurements in lumbar intervertebral discs. Proc SPIE. 1999;3570:115–122. doi: 10.1117/12.336921. [DOI] [Google Scholar]
- 8.Hutton WC, Ganey TM, Elmer WA, Kozlowska EE, Ugbo JL, Doh ES, Whitesides TE. Does long-term compressive loading on the intervertebral disc cause degeneration. Spine. 2000;25(23):2993–3004. doi: 10.1097/00007632-200012010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Iatridis JC, Mente PL, Stokes IA, Aronsson DD, Alini M. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine. 1999;24:996–1002. doi: 10.1097/00007632-199905150-00013. [DOI] [PubMed] [Google Scholar]
- 10.Kroeber MW, Unglaub F, Wang H, Schmid C, Thomsen M, Nerlich A, Richter W. New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutical strategies to stimulate disc regeneration. Spine. 2002;23:2684–2690. doi: 10.1097/00007632-200212010-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kroeber M, Unglaub F, Guehring T, Campana M, Carstens C. Poster presentation ESS Prag 2003, award “best poster in basic science”. Eur Spine J. 2003;12(suppl1):S1–S79. doi: 10.1007/s00586-003-0587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroeber M, Unglaub F, Guehring T, Nerlich A, Tamer H, Lotz J, Carstens C. Effects of controlled dynamic disc distraction on degenerated intervertebral discs: an in vivo study on the rabbit lumbar spine model. Spine. 2005;30(2):181–187. doi: 10.1097/01.brs.0000150487.17562.b1. [DOI] [PubMed] [Google Scholar]
- 13.Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Volvo award winner in biomechanical studies. Compression-induced degeneration of the intervertebral disc: An in vivo mouse model and finite-element study. Spine. 1998;23:2493–2506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Nachemson AL. Towards a better understanding of low-back pain: a review of the mechanics of the lumbar disc. Rheumatoid Rehabil. 1975;14(3):129–143. doi: 10.1093/rheumatology/14.3.129. [DOI] [PubMed] [Google Scholar]
- 15.Panjabi M, Brown M, Lindahl S, Irstam L, Hermens M. Intrinsic disc pressures a measure of integrity of the lumbar spine. Spine. 1988;13(8):913–917. doi: 10.1097/00007632-198808000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Phillips FM, Reuben J, Wetzel FT (2002) Intervertebral disc degeneration adjacent to a lumbar fusion. An experimental rabbit model. J Bone Joint Surg Br 3;84(2):289–294 [DOI] [PubMed]
- 17.Pospiech J, Wilke HJ, Claes LE, Stolke D. Intradiscal pressure forces on cervical intervertebral discs in physiologic and pathologic conditions. In vitro study. Langenbecks Arch Chir. 1996;381(6):303–308. doi: 10.1007/BF00191309. [DOI] [PubMed] [Google Scholar]
- 18.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase. Spine. 2000;23:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 19.Rubinstein R. Simulation and the Monte Carlo Method. New York: Wiley; 1981. [Google Scholar]
- 20.Sasaki M, Takahashi T, Miyahara K, Hirose AT. Effects of chondroitinase ABC on intradiscal pressure in sheep: an in vivo study. Spine. 2001;26:463–468. doi: 10.1097/00007632-200103010-00008. [DOI] [PubMed] [Google Scholar]
- 21.Sato K, Kikuchi S, Yonezawa T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine. 1999;23:2468–2474. doi: 10.1097/00007632-199912010-00008. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Nagata K, Hirohashi T. Intradiscal pressure after repeat intradiscal injection of hypertonic saline:an experimental study. Eur Spine J. 2002;11(1):52–56. doi: 10.1007/s005860100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sieve J, Baird P, Jeziorsk M, Watkins A, Hoyland JA, Freemont AJ. Expression of chondrocyte markers by cells of normal and degenerative intervertebral discs. J Clin Pathol Mol Pathol. 2002;55:91–97. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoll TM, Dubois G, Schwarzenbach O. The dynamic neutralization system for the spine: a multi-center study of a novel non-fusion system. Eur Spine J. 2002;8(11Suppl2):S170–S178. doi: 10.1007/s00586-002-0438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swanson KE, Lindsey DP, Hsu KY, Zucherman JF, Yerby SA. The effect of an interspinous implantat on intervertebral disc pressure. Spine. 2003;28(1):26–32. doi: 10.1097/00007632-200301010-00008. [DOI] [PubMed] [Google Scholar]
- 26.Unglaub Effects of unisegmental dynamic disc compression on adjacent. 2005;segments:an. doi: 10.1007/s00586-004-0800-7. [DOI] [PubMed] [Google Scholar]
- 27.Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37(3):329–337. doi: 10.1016/S0021-9290(03)00290-2. [DOI] [PubMed] [Google Scholar]
- 28.Weiler C, Nerlich A, Zipperer J, Bachmeier BE, Boos N. 2002 SSE award competition in basic science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinhoffer SL, Guyer RD, Herbert M, Griffith SL. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine. 1995;20:526–531. doi: 10.1097/00007632-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 30.Wilke H, Neef P, Hinz B, Seidel H, Claes L. Intradiscal pressure together with anthropometric data – a data set for validation of models. Clin Biomech. 2001;16(Suppl1):111–126. doi: 10.1016/S0268-0033(00)00103-0. [DOI] [PubMed] [Google Scholar]
- 31.Zöfel P, Bühl A. SPSS Version 10; Einführung in die moderne Datenanlyse unter Windows. London: Addison Wesley Longman Publishing Group; 2000. [Google Scholar]