Abstract
Recognition of pathogen attack or elicitation with pathogen-associated molecular patterns (PAMPs) leads to defense signaling that includes activation of the three mitogen-activated protein kinases (MPKs), MPK3, MPK4 and MPK6 in Arabidopsis. Recently, we demonstrated the activation of a fourth MPK, MPK11, after treatment with flg22, a 22 amino acid PAMP derived from bacterial flagellin. Here, we extended the study by examining elicitation with two other PAMPs, elf18 (derived from bacterial elongation factor EF-Tu) and ch8 (N-acetylchitooctaose derived from fungal chitin). Both PAMPs led to rapid MPK11 transcript accumulation and increased MPK11 kinase activity, suggesting that multiple PAMPs (or stresses) can activate MPK11. However, probably due to functional redundancies, bacteria-induced phytoalexin accumulation does not absolutely require MPK11.
Keywords: MAP kinase, defense, pathogen-associated molecular pattern, signal transduction
Adaptive processes enable plants to cope with changing environmental conditions. Various abiotic or biotic stresses lead to activation of tightly regulated signaling networks that determine the plant’s response. Early steps in the signaling network include mitogen-activated protein kinase (MPK) cascades that typically comprise of at least three hierarchically organized kinases, a MPK, a MPK kinase and a MPK kinase kinase.1,2 Upon phosphorylation, the activities and/or functions of MPK substrates are altered to effect the appropriate responses.1 Plant innate immunity studies identified the Arabidopsis MPKs, MPK3 and 6, as positive regulators of defense responses induced by PAMP and pathogen treatment,3-6 whereas MPK4 was shown to be a negative regulator of salicylic acid-dependent responses.7
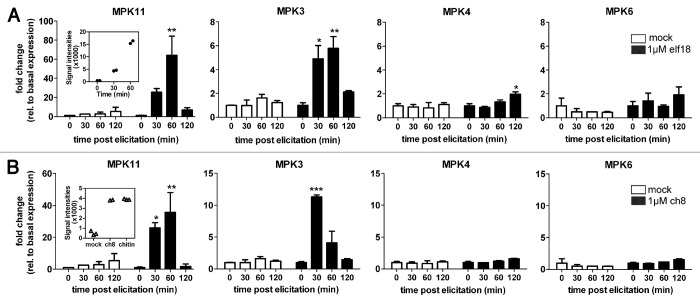
Like its homolog MPK4, MPK11 was reported to be involved in cytokinesis8 and to interact with the upstream MPK kinases, MKK1 and MKK2 in yeast two-hybrid experiments.9 In a previous study, we showed transient accumulation of MPK11 transcripts and increased MPK11 activity upon flg22 elicitation.10 We now extended our analyses to additional PAMPs. Like MPK3 [11], MPK11 transcripts accumulated transiently upon treatment of seedlings with the bacterial elf18 peptide or the fungal cell wall-derived ch8 fragment (Fig. 1A and B). By contrast, in the case of the other two MPKs, no (for MPK6) or only a very marginal increase (at 120 min for MPK4) was detected. These data corroborate our results obtained with flg22 treatment.10 Thus, in agreement with microarray expression profiles from Genevestigator database12 (see inserts in Figure 1A and B), MPK11 is responsive to several PAMPs.
Figure 1. Expression of MPK3, 4, 6 and 11 upon elicitation with PAMPs. (A, B) Arabidopsis thaliana Col-0 seedlings were grown in liquid MS medium in 24-well microtiter plates (10 seeds per well) as described.16 Seedlings were either mock-treated with an equivalent volume of medium (white bars) or elicited with 1 µM elf18 (A) and ch8 (B) (black bars). Samples were taken at the indicated time post elicitation. Total RNA isolation, cDNA synthesis and quantitative real-time PCR were performed as described.17 Expression levels are depicted as fold changes with respect to the basal expression level at time point zero. Statistical significance of expression compared with the basal levels are indicated (*p < 0.05, **p < 0.01, ***p < 0.001). The inserts in the first panels depict the MPK11 microarray signal intensities of corresponding experiments from microarray depositories (Array Express E-MEXP-547 for elf26 treatment and GEO GSM48125 for treatment with chitin prepared from crab-shells or N-acetylchitooctaose, ch8).
To study MPK11 activation upon elf18 or ch8 treatment, haemagglutinin (HA)-tagged MPK11 under the control of the Cauliflower mosaic virus (CaMV) 35S promoter (pUGW15 vector13) was transiently expressed in Arabidopsis mesophyll protoplasts and the immuno-precipitated HA-MPK11 protein was used in kinase assays. This revealed MPK11 activation upon elf18 and chitin elicitation (Fig. 2A and B). Western blot with α-HA antibody (HA.11 monoclonal antibody clone 16B12, Eurogentec) confirmed comparable expression of HA-MPK11, so that lack of kinase activity (in the water controls) is not due to absence of the protein (Fig. 2C).
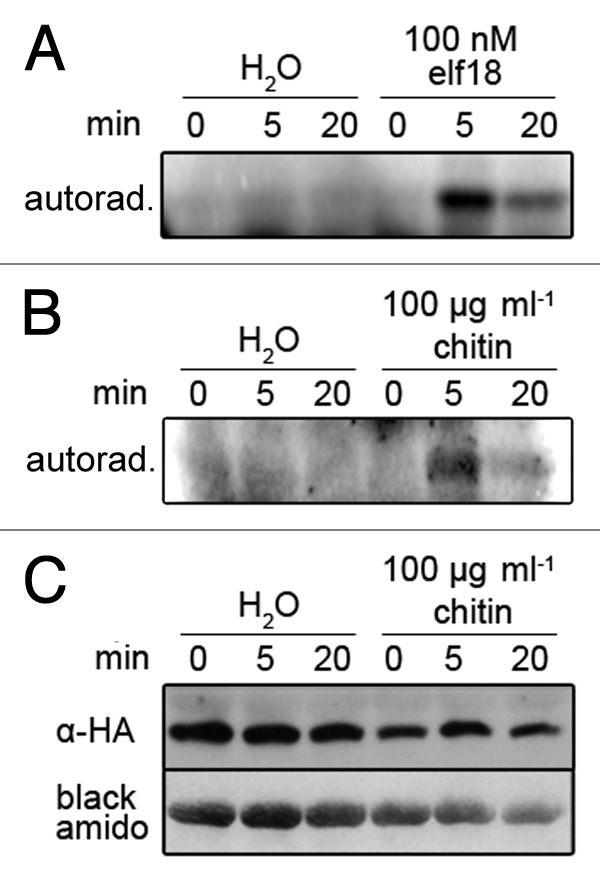
Figure 2. PAMP induced MPK11 activity. Arabidopsis mesophyll protoplast preparation and transformation with pUGW15-MPK11 were performed as described.16,18 (A, B) Protoplasts were elicited with elf18, chitin (from crab-shells) or water as a control, and harvested by centrifugation (short pulse, 15000 g) at the indicated time points. Immunoprecipitation of HA-MPK11 (α-HA; Eurogentec, Cologne, Germany) and kinase assays with myelin basic protein (MBP) as artificial substrate were performed as described19,20 using 50 µg total protein per sample. The autoradiograms show the radioactive signals for the phosphorylated MBP. (C) To validate equal expression of MPK11 protein, a representative western blot (α-HA) was performed with 20 µg of proteins from the transfected protoplast (from the chitin-treated samples shown in B).
To further address the impact of MPK11 on the defense response, levels of camalexin, a major phytoalexin of Arabidopsis,14 were measured in the mpk11 mutant (SALK_049352) and Col-0 wild type plants after infection with Pseudomonas syringae pv maculicola (Pma ES4326). As an additional control, the pad4–1 (phytoalexin-deficient 4–1) mutant was included in the analyses, which was shown to be impaired in camalexin accumulation.15 No statistically significant difference between wild type and mpk11 mutant plants was detected, whereas pad4–1 plants accumulated significantly less camalexin (Fig. 3).
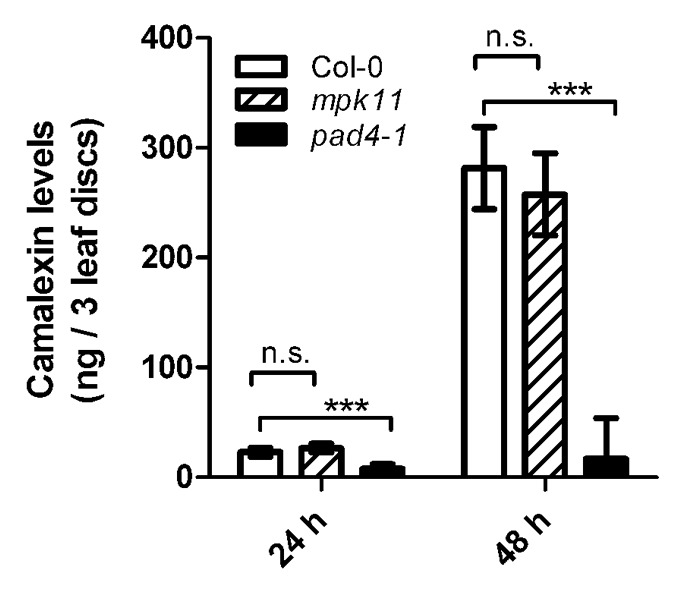
Figure 3. Camalexin accumulation. Four week old plants (Col-0, mpk11, pad4–1) were inoculated with virulent Pma ES4326 (OD600 = 0.002) and harvested 24 and 48 h later. Camalexin was extracted as described previously6,21 and quantified by comparison with standards containing known amounts of camalexin. Two independent biological experiments each consisting of 8 biological replicates were performed (each biological replicate contained 3 leaf discs from 2 inoculated leaves from 1 plant). Data were combined using a mixed linear model and the statistical significances are indicated (n.s. = not significant, *** = p < 0.001).
In summary, the current data indicate that MPK11 expression and kinase activity are stimulated by several PAMPs. MPK11 is highly homologous to MPK4 but its stress-induced expression profile is more similar to that of MPK3. Previously, we report compromised development of the mpk4 mpk11 double mutants and failure to obtain mpk3 mpk11 or mpk6 mpk11 double mutants. Taken together, MPK11 presumably has some functional redundancies with other MPKs in both developmental and defense processes, which explains why the single mpk11 mutant does not exhibit strong phenotypes (Fig. 3).10 Nevertheless, the discovery of MPK11 as a fourth PAMP-activated MPK accentuates the need to include it in future studies of MPK function in defense signaling.
Acknowledgment
Funding for our research is provided by programs from the German Research Foundation (SFB648), the State of Saxony-Anhalt (Graduate Program, W21040908) and the Federal Ministry of Education and Research (ProNET-T3, 03ISO2211B). G.B. and J. G are supported by a grant from the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, US. Department of Energy (DE-FG02–09ER15670).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/21323
References
- 1.Colcombet J, Hirt H. Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem J. 2008;413:217–26. doi: 10.1042/BJ20080625. [DOI] [PubMed] [Google Scholar]
- 2.Sinha AK, Jaggi M, Raghuram B, Tuteja N. Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal Behav. 2011;6:196–203. doi: 10.4161/psb.6.2.14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–83. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 4.Galletti R, Ferrari S, De Lorenzo G. Arabidopsis MPK3 and MPK6 play different roles in basal and oligogalacturonide- or flagellin-induced resistance against Botrytis cinerea. Plant Physiol. 2011;157:804–14. doi: 10.1104/pp.111.174003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han L, Li GJ, Yang KY, Mao GH, Wang RQ, Liu YD, et al. Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea-induced ethylene production in Arabidopsis. Plant J. 2010;64:114–27. doi: 10.1111/j.1365-313X.2010.04318.x. [DOI] [PubMed] [Google Scholar]
- 6.Ren DT, Liu YD, Yang KY, Han L, Mao GH, Glazebrook J, et al. A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci U S A. 2008;105:5638–43. doi: 10.1073/pnas.0711301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell. 2000;103:1111–20. doi: 10.1016/S0092-8674(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 8.Kosetsu K, Matsunaga S, Nakagami H, Colcombet J, Sasabe M, Soyano T, et al. The MAP kinase MPK4 is required for cytokinesis in Arabidopsis thaliana. Plant Cell. 2010;22:3778–90. doi: 10.1105/tpc.110.077164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JS, Huh KW, Bhargava A, Ellis BE. Comprehensive analysis of protein-protein interactions between Arabidopsis MAPKs and MAPK kinases helps define potential MAPK signalling modules. Plant Signal Behav. 2008;3:1037–41. doi: 10.4161/psb.3.12.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bethke G, Pecher P, Eschen-Lippold L, Tsuda K, Katagiri F, Glazebrook J, et al. Activation of the Arabidopsis thaliana mitogen-activated protein kinase MPK11 by the flagellin-derived elicitor peptide, flg22. Mol Plant Microbe Interact. 2012;25:471–80. doi: 10.1094/MPMI-11-11-0281. [DOI] [PubMed] [Google Scholar]
- 11.Wan J, Zhang S, Stacey G. Activation of a mitogen-activated protein kinase pathway in Arabidopsis by chitin. Mol Plant Pathol. 2004;5:125–35. doi: 10.1111/j.1364-3703.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 12.Hruz, T., O. Laule, G. Szabo, F. Wessendorp, S. Bleuler, L. Oertle, et al., Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics, 2008; 2008: 420747. [DOI] [PMC free article] [PubMed]
- 13.Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng. 2007;104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- 14.Ahuja I, Kissen R, Bones AM. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012;17:73–90. doi: 10.1016/j.tplants.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Glazebrook J, Zook M, Mert F, Kagan I, Rogers EE, Crute IR, et al. Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics. 1997;146:381–92. doi: 10.1093/genetics/146.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D. Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J. 2011;68:100–13. doi: 10.1111/j.1365-313X.2011.04671.x. [DOI] [PubMed] [Google Scholar]
- 17.Eschen-Lippold L, Landgraf R, Smolka U, Schulze S, Heilmann M, Heilmann I, et al. Activation of defense against Phytophthora infestans in potato by down-regulation of syntaxin gene expression. New Phytol. 2012;193:985–96. doi: 10.1111/j.1469-8137.2011.04024.x. [DOI] [PubMed] [Google Scholar]
- 18.Bethke G, Unthan T, Uhrig JF, Pöschl Y, Gust AA, Scheel D, et al. Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis thaliana via ethylene signaling. Proc Natl Acad Sci U S A. 2009;106:8067–72. doi: 10.1073/pnas.0810206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahlfors R, Macioszek V, Rudd J, Brosché M, Schlichting R, Scheel D, et al. Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. 2004;40:512–22. doi: 10.1111/j.1365-313X.2004.02229.x. [DOI] [PubMed] [Google Scholar]
- 20.Ligterink W, Kroj T, zur Nieden U, Hirt H, Scheel D. Receptor-mediated activation of a MAP kinase in pathogen defense of plants. Science. 1997;276:2054–7. doi: 10.1126/science.276.5321.2054. [DOI] [PubMed] [Google Scholar]
- 21.Glazebrook J, Ausubel FM. Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc Natl Acad Sci U S A. 1994;91:8955–9. doi: 10.1073/pnas.91.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]