Abstract
UDP-N-acetylglucosamine (UDP-GlcNAc) is an important sugar nucleotide used as a precursor of cell wall components in bacteria, and as a substrate in the synthesis of oligosaccharides in eukaryotes. In bacteria UDP-GlcNAc is synthesized from the glycolytic intermediate D-fructose-6-phosphate (fructose-6P) by four successive reactions catalyzed by three enzymes: glucosamine-6-phosphate synthase (GlmS), phosphoglucosamine mutase (GlmM) and the bi-functional enzyme glucosamine-1-phosphate acetyltransferase/ N-acetylglucosamine-1-phosphate uridyltransferase (GlmU). We have previously reported a metabolic engineering strategy in Lactobacillus casei directed to increase the intracellular levels of UDP-GlcNAc by homologous overexpression of the genes glmS, glmM and glmU. One of the most remarkable features regarding the production of UDP-GlcNAc in L. casei was to find multiple regulation points on its biosynthetic pathway: (1) regulation by the NagB enzyme, (2) glmS RNA specific degradation through the possible participation of a glmS riboswitch mechanism, (3) regulation of the GlmU activity probably by end product inhibition and (4) transcription of glmU.
Keywords: UDP-N-acetylglucosamine, Lactobacillus, glucosamine-6-phosphate synthase, phosphoglucosamine mutase, glucosamine-1-phosphate acetyltransferase, N-acetylglucosamine-1-phosphate uridyltransferase
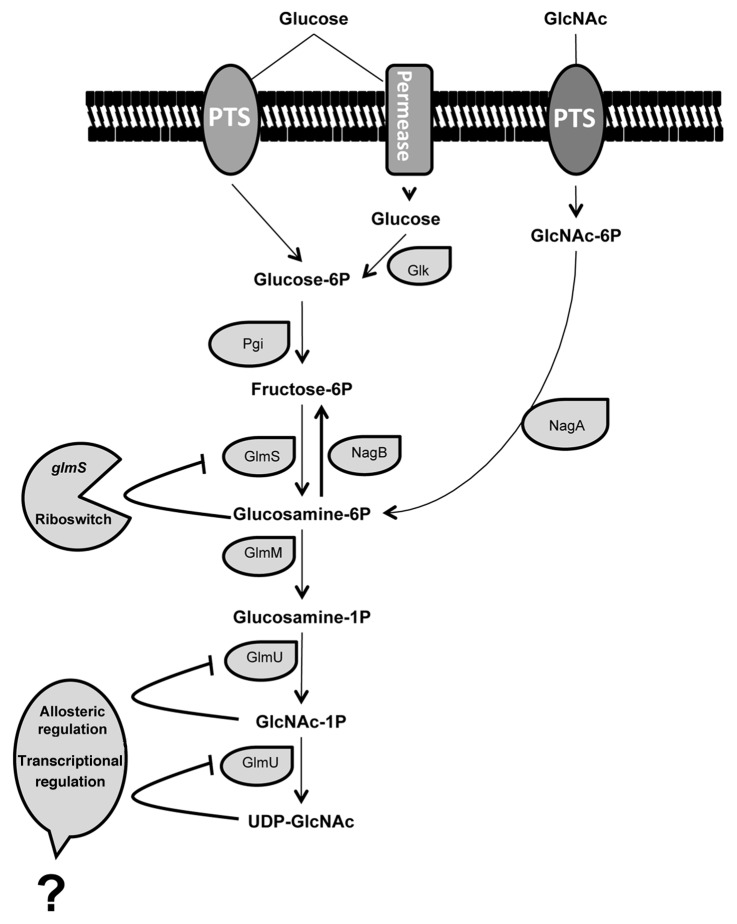
Oligosaccharides, which form part of glycoconjugates on the cell surface or are soluble as in the human milk, play active roles in numerous cell-cell interactions. They are also used as anchoring molecules for pathogens, antibodies and hormones.1-4 The synthesis of those carbohydrates is catalyzed by glycosyltransferases that use activated sugars, such as UDP-N-acetylglucosamine (UDP-GlcNAc), UDP-glucose and UDP-galactose. The difficulties in obtaining oligosaccharides using chemical or enzymatic synthesis, which require expensive UDP-sugars as substrate, have limited the research and its possible applications. One way to overcome this limitation is to use a whole-cell system to allow the regeneration in situ of sugar nucleotides. UDP-GlcNAc is synthesized from the glycolytic intermediate D-fructose-6-phosphate (fructose-6P) by four successive reactions catalyzed by the enzymes glucosamine-6-phosphate synthase (GlmS),5 phosphoglucosamine mutase (GlmM)6,7 and the bi-functional enzyme glucosamine-1-phosphate acetyltransferase/N-acetylglucosamine-1-phosphate uridyltransferase (GlmU)8-10 (Fig. 1).
Figure 1. Schematic representation of proposed pathways and regulation mechanisms involved in UDP-N-acetylglucosamine synthesis by Lactobacillus casei. PTS, phosphoenol-pyruvate phosphotransferase system; GlcNAc, N-acetylglucosamine; Glk, glucokinase; Pgi, phosphoglucose isomerase; NagA, GlcNAc-6P deacetylase; NagB, glucosamine-6-phosphate deaminase; GlmS, glucosamine-6-phosphate synthase; GlmM, phosphoglucosamine mutase; GlmU, glucosamine-1-phosphate acetyltransferase/N-acetylglucosamine-1-phosphate uridyltransferase. Bold lines represent the regulatory features of the UDP-GlcNAc pathway.
We have recently overexpressed the three enzymes of the UDP-GlcNAc biosynthetic pathway (GlmS, GlmM and GlmU) in the probiotic bacteria Lactobacillus casei BL23, and a recombinant L. casei PL33 strain with about a 4-fold increase of the UDP-GlcNAc pool has been constructed. This engineered strain might be a good candidate as a cell factory for the production of oligosaccharides.11 Interestingly only the L. casei PL33 strain, that simultaneously overexpressed two of the genes (glmMS), cloned with their intergenic region, was able to accumulate high amounts of UDP-GlcNAc. The other three recombinant strains constructed, PL30 (glmS), PL31 (glmM) and PL32 (glmU), showed no increase or an increase of about 1.5 times on the UDP-GlcNAc level. The measurements of the specific activities of each enzyme in the recombinant L. casei strains as well as the RT-qPCR results show that the pathway for the production of UDP-GlcNAc is very tightly regulated in L. casei.
Regulation by NagB Enzyme
Cell extracts of strain PL30 (glmS) showed about 12 times more GlmS activity than the control strain.11 However, this high increase was not reflected in the UDP-GlcNAc concentration, which remains at the same level as that of the control strain. This result might be explained by the activity of the enzyme glucosamine-6-phosphate deaminase (NagB) that catalyzes the opposite reaction of GlmS, thus the conversion of glucosamine-6P into fructose-6P. Accordingly, a gene LCABL_31070, encoding a presumed NagB, is present in the L. casei BL23 genome.12 Recently, we have assayed the UDP-GlcNAc production in all the engineered strains described above cultured on GlcNAc as the carbon source. The UDP-GlcNAc production in these experimental conditions was compared with the production on glucose (Table 1). The results showed that the growth on GlcNAc did not result in an important increment in the UDP-GlcNAc pool, suggesting that the NagB activity directed the glucosamine-6P obtained from the GlcNAc catabolism to fructose-6P. Those observations suggested that the NagB enzyme plays an important role in the control of the carbon flux in the UDP-GlcNAc biosynthetic pathway.
Table 1. UDP-N-acetylglucosamine levels in pmol/mg of protein in the Lactobacillus casei strains PL27, PL30, PL32 and PL33a.
| Strainsb | PL27 (pNG8048E) |
PL30 (pGlmS) |
PL31 (pGlmM) |
PL32 (pGlmU) |
PL33 (pGlmMS) |
|---|---|---|---|---|---|
| Glucose |
821.29 |
799.82 |
877.66 |
1289.54 |
3181.43 |
| GlcNAc | 839.02 | 993.03 | 834.82 | 1391.06 | 3935.13 |
a UDP-N-acetylglucosamine analysis were performed as previously described11; bStrains were cultured in MRS fermentation medium with 0.5% glucose or 0.5% N-acetylglucosamine (GlcNAc).
Regulation by glmS Riboswitch
Numerous bacterial genes of related function are organized in operons and transcribed as polycistronic mRNA to guarantee the coordinate expression of the individual genes. However, post-transcriptional modification of such mRNA can modulate the gene’s translational expression under specific environmental conditions. This is the case for the cis-acting regulatory RNAs called riboswitches, including the glmS ribozyme that uses glucosamine-6P as a cofactor and activates self-cleavage of the bacterial rybozyme, which is part of the mRNA coding for GlmS.13 The glmS ribozymes are based in conserved structures more than in conserved sequences and they are highly specific for glucosamine-6P.13-18 The glmS riboswitch was first described in Bacillus subtilis13 but since then it has been found in many other bacterial groups including some Lactobacillus strains.14 In L. casei we showed by reverse transcriptase PCR analysis using total RNA isolated from strain BL23 (wt) grown on glucose as carbon source, that both genes, glmS and glmM, are transcribed as a single mRNA (Fig. 2). Curiously, using RT-qPCR analysis and RNA isolated from BL23 strain grown on GlcNAc as carbon source, the transcript levels of glmS, but not the transcript levels of glmM, decrease 21 times with respect to BL23 cultured in glucose. In L. casei GlcNAc is probably transported and phosphorylated to GlcNAc-6P, which is deacetyled to glucosamine-6P by a deacetylase. This is in agreement with the presence of a gene, LCABL_20280, in the L. casei BL23 genome,12 that encodes a presumed GlcNAc-6P deacetylase (NagA). The glucosamine-6P produced in an independent manner from the GlmS activity could trigger the glmS riboswich hypothetically contained in the glmM-glmS intergenic region and degrade glmS RNA.
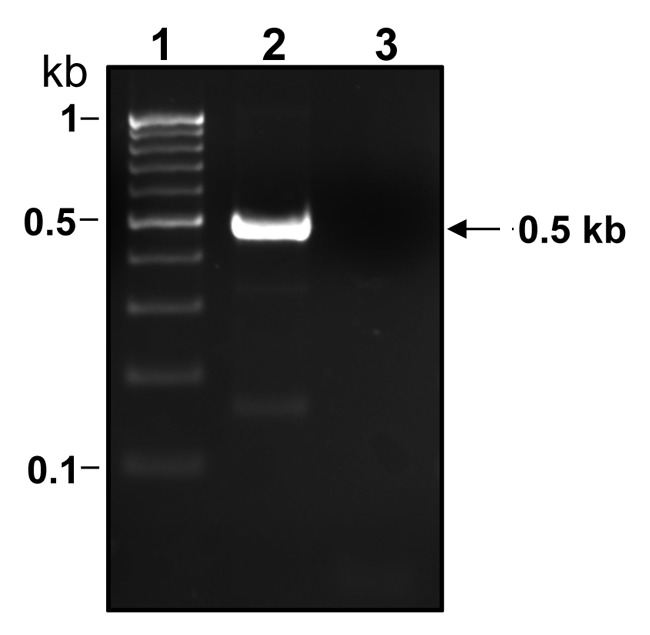
Figure 2. Agarose gel showing a RT-PCR band obtained with RNA isolated from Lactobacillus casei BL23 (wt) cultured on MRS fermentation medium with 0.5% glucose. Total RNA was used in RT reactions using the Maxima First strand cDNA Synthesis Kit (Fermentas) with Maxima Enzyme Mix (lane 2) or without Maxima Enzyme Mix (lane 3). The cDNAs obtained were used in PCRs with primers glmM4 (CACTGAACCTTTGTTGCGG) and glmS1 (ACTTCTCTAATCCCTTAAGC). Size standard markers are shown in lane 1. The size of the fragment obtained is marked on the right.
GlmU Regulation
The PL33 (glmMS) strain produces about four times more UDP-GlcNAc than the control strain. PL33 showed a 6.47 and 3.87 fold increase in GlmM and GlmS specific activity respectively.11 Since the PL30 (glmS) strain showed about 12 times more GlmS activity than the control strain, but the same UDP-GlcNAc levels, the GlmM seems to be the key enzyme to increment the UDP-GlcNAc pool in L. casei. The glucosamine-1P produced by the GlmM activity is converted to UDP- GlcNAc by two enzymatic steps conducted by the GlmU enzyme. In the first step, GlmU catalyzes the CoA-dependent acetylation of glucosamine-1P to form GlcNAc-1P. This sugar-P, in a subsequent reaction with UTP, is converted in UDP-GlcNAc. Surprisingly in the L. casei PL33 (glmMS) strain, which produces about four times more UDP-GlcNAc than the control strain, the GlmU specific activity was reduced to 38% of the control strain.11 This decrease in the GlmU activity might be explained by two possible mechanims: (1) allosteric inhibition by end product as it has been previously reported to occur with the GlmU enzyme from Escherichia coli9; (2) transcriptional regulation as we have previously showed that the transcript levels of glmU in the PL33 (glmMS) strain decreased 8-fold compared with the control strain.
Final Remarks
The production of UDP-GlcNAc is tightly regulated in L. casei BL23. This regulation probably takes place at 4 different levels of the UDP-GlcNAc biosynthetic pathway (Fig. 1). The tight regulation is in agreement with the importance of the production of UDP-GlcNAc to build the cell wall components in L. casei. This addendum has discussed the observations that point to the possible regulation mechanisms but future work should provide additional evidence to confirm these remarkable regulatory events.
Acknowledgments
This work was financed by funds of the Spanish Ministry for Science and Innovation (MICINN)/FEDER through Projects AGL2007–63060 and Consolider Fun-c-Food CSD2007–00063. J.R.D. was supported by a JAE-doc contract from CSIC.
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/21271
References
- 1.Appelmelk BJ, van Die I, van Vliet SJ, Vandenbroucke-Grauls CM, Geijtenbeek TB, van Kooyk Y. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J Immunol. 2003;170:1635–9. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 2.Levander L, Gunnarsson P, Grenegård M, Rydén I, Påhlsson P. Effects of alpha1-acid glycoprotein fucosylation on its Ca2+ mobilizing capacity in neutrophils. Scand J Immunol. 2009;69:412–20. doi: 10.1111/j.1365-3083.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- 3.Misonou Y, Shida K, Korekane H, Seki Y, Noura S, Ohue M, et al. Comprehensive clinico-glycomic study of 16 colorectal cancer specimens: elucidation of aberrant glycosylation and its mechanistic causes in colorectal cancer cells. J Proteome Res. 2009;8:2990–3005. doi: 10.1021/pr900092r. [DOI] [PubMed] [Google Scholar]
- 4.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- 5.Badet B, Vermoote P, Le Goffic F. Glucosamine synthetase from Escherichia coli: kinetic mechanism and inhibition by N3-fumaroyl-L-2,3-diaminopropionic derivatives. Biochemistry. 1988;27:2282–7. doi: 10.1021/bi00407a006. [DOI] [PubMed] [Google Scholar]
- 6.Mengin-Lecreulx D, van Heijenoort J. Characterization of the essential gene glmM encoding phosphoglucosamine mutase in Escherichia coli. J Biol Chem. 1996;271:32–9. doi: 10.1074/jbc.271.1.32. [DOI] [PubMed] [Google Scholar]
- 7.Jolly L, Ferrari P, Blanot D, Van Heijenoort J, Fassy F, Mengin-Lecreulx D. Reaction mechanism of phosphoglucosamine mutase from Escherichia coli. Eur J Biochem. 1999;262:202–10. doi: 10.1046/j.1432-1327.1999.00373.x. [DOI] [PubMed] [Google Scholar]
- 8.Mengin-Lecreulx D, van Heijenoort J. Identification of the glmU gene encoding N-acetylglucosamine-1-phosphate uridyltransferase in Escherichia coli. J Bacteriol. 1993;175:6150–7. doi: 10.1128/jb.175.19.6150-6157.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mengin-Lecreulx D, van Heijenoort J. Copurification of glucosamine-1-phosphate acetyltransferase and N-acetylglucosamine-1-phosphate uridyltransferase activities of Escherichia coli: characterization of the glmU gene product as a bifunctional enzyme catalyzing two subsequent steps in the pathway for UDP-N-acetylglucosamine synthesis. J Bacteriol. 1994;176:5788–95. doi: 10.1128/jb.176.18.5788-5795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hove-Jensen B. Identification of tms-26 as an allele of the gcaD gene, which encodes N-acetylglucosamine 1-phosphate uridyltransferase in Bacillus subtilis. J Bacteriol. 1992;174:6852–6. doi: 10.1128/jb.174.21.6852-6856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Díaz J, Rubio-Del-Campo A, Yebra MJ. Metabolic engineering of Lactobacillus casei for production of UDP-N-acetylglucosamine. Biotechnol Bioeng. 2012;109:1704–12. doi: 10.1002/bit.24475. [DOI] [PubMed] [Google Scholar]
- 12.Mazé A, Boël G, Zúñiga M, Bourand A, Loux V, Yebra MJ, et al. Complete genome sequence of the probiotic Lactobacillus casei strain BL23. J Bacteriol. 2010;192:2647–8. doi: 10.1128/JB.00076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428:281–6. doi: 10.1038/nature02362. [DOI] [PubMed] [Google Scholar]
- 14.McCown PJ, Roth A, Breaker RR. An expanded collection and refined consensus model of glmS ribozymes. RNA. 2011;17:728–36. doi: 10.1261/rna.2590811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrick JE, Corbino KA, Winkler WC, Nahvi A, Mandal M, Collins J, et al. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proc Natl Acad Sci U S A. 2004;101:6421–6. doi: 10.1073/pnas.0308014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen JA, McCarthy TJ, Soukup GA, Soukup JK. Backbone and nucleobase contacts to glucosamine-6-phosphate in the glmS ribozyme. Nat Struct Mol Biol. 2006;13:517–23. doi: 10.1038/nsmb1094. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy TJ, Plog MA, Floy SA, Jansen JA, Soukup JK, Soukup GA. Ligand requirements for glmS ribozyme self-cleavage. Chem Biol. 2005;12:1221–6. doi: 10.1016/j.chembiol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Wakeman CA, Winkler WC, Dann CE., 3rd Structural features of metabolite-sensing riboswitches. Trends Biochem Sci. 2007;32:415–24. doi: 10.1016/j.tibs.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]