Abstract
We recently reported that anti-inflammatory macrophages contribute to the initiation of colorectal carcinogenesis in IBD patients by inducing epithelial-mesenchymal-transition associated alterations in colonic epithelial cells. In this process, TGFβ1 dependent upregulation of the adhesion molecule L1CAM is one key event, paving the way to colitis associated tumorigenesis and metastatic spread.
Keywords: CD171, colitis associated cancer, colorectal cancer, epithelial-mesenchymal transition, L1CAM, macrophages, Nrf2
Besides genetic alterations in sporadic colon cancer, the persistent inflammation during inflammatory bowel diseases (IBD) is regarded as another high-risk factor of colorectal cancer (CRC). Accordingly, the inflammatory microenvironment being characterized by an elevated number of lamina propria macrophages has a pivotal role in colorectal carcinogenesis.1 Several mechanisms and signalling molecules have been identified by which pro-inflammatory macrophages can induce certain tumorigenic alterations in enterocytes, e.g. the oxidative-stress induced activity of the transcription factor Nrf2.2 Interestingly, the inflamed tissues of IBD patients also comprise a variety of putative anti-inflammatory cells and mediators, e.g. Transforming growth factor-beta1 (TGF-β1) contributing to colitis associated carcinogenesis.3 This seems contradictory at first glance, but more and more ongoing studies unveil the underlying mechanisms. Thus, we recently demonstrated that anti-inflammatory macrophages promote malignant transformation of epithelial cells by upregulating the cell adhesion molecule L1CAM (CD171) in a TGF-β1 and Slug dependent fashion during epithelial-mesenchymal-transition (EMT).4 Moreover, this mechanism is also involved in early carcinogenesis5 - including CRC6 - and therapy resistance,7 so that one could expect L1CAM contributing to tumorigenic alterations in the inflamed colon during IBD. In fact, a disease associated and progressive L1CAM expression in enterocytes was detected by immunohistochemistry in tissue samples from Ulcerative colitis and Crohn’s disease patients, but not in non-inflamed colon tissues.4 L1CAM expression patterns correlated with the appearance of infiltrating macrophages, as shown by the high abundance of L1CAM expressing enterocytes in direct proximity to macrophages.4
Using a transwell coculture system with the colonocyte cell line NCM460, we verified an L1CAM inducing effect by anti-inflammatory, M2-polarized macrophages generated in vitro from M-CSF treated monocytes.4 In accordance with TGF-β1 secretion by anti-inflammatory macrophages—a hallmark of this macrophage subset—L1CAM expression was abrogated in cocultured NCM460 cells by blockade of TGF-β1 signalling. Whilst TGF-β1 induced L1CAM expression in NCM460 colonocytes was insensitive to smad2/3 blockade, abrogation of JNK and Slug induction by TGF-β1 diminished L1CAM expression.4 This Slug dependency of anti-inflammatory macrophage induced L1CAM expression could be regarded as an integral part of the TGF-β1 induced EMT. Accordingly, NCM460 colonocytes acquire a migratory and less apoptotic phenotype in a L1CAM dependent fashion.4
By clearly demonstrating a link between TGF-β1 and the L1CAM dependent EMT associated alterations in colonic epithelial cells, our recent study provides a novel mechanism by which an anti-inflammatory microenvironment during chronic inflammation promotes malignant transformation in intestinal epithelial cells and the onset of colorectal carcinogenesis.4 Intriguingly, L1CAM dependent EMT is induced by anti-inflammatory macrophages regarded as one major source of TGF-β1 secretion in IBD tissues.3 The appearance of anti-inflammatory mediators during chronic inflammation probably reflects the body’s attempt to cope with the inflammatory burden during IBD, but this creates also a microenvironment favouring the initiation of malignant transformation of the diseased colonic epithelium. Since L1CAM has been recognized as to have an important role in early carcinogenesis and since L1CAM expression correlates with metastatic spread and poor prognosis of tumor patients - including those with CRC8 - L1CAM could be used as early diagnostic and predictive marker in colorectal carcinogenesis, particularly in patients with IBD as risk factor. In addition, L1CAM may serve as target in novel strategies of anti-cancer therapy, e.g. in combination with common cytostatic drugs such as 5-fluoruracil or irinotecan. Supporting this idea, blocking L1CAM antibodies improved the effects by anti-cancer drug response rates of certain types of cancer9 and it would be worth to test this strategy also in CRC patients.
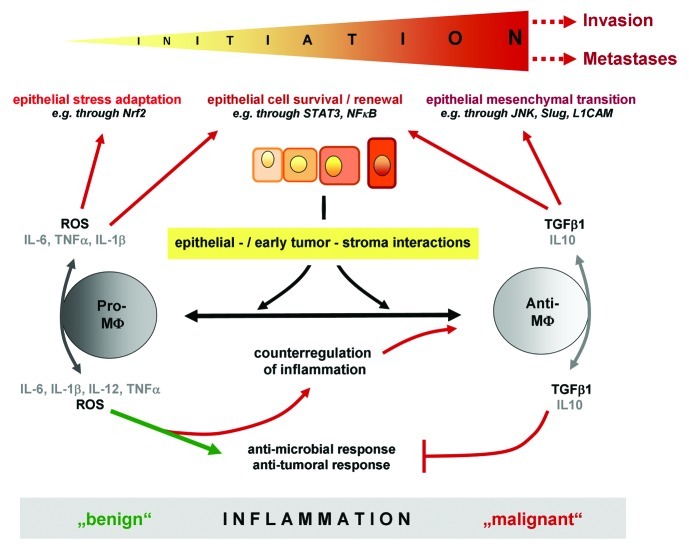
The observation that anti-inflammatory macrophages induce EMT associated alterations in colonic epithelial cells4 is compatible with the role of tumor associated macrophages regarded as M2-polarized macrophages. Moreover, recent reports demonstrated a correlation between tumor infiltration of M2-polarized macrophages and a poor prognosis for cancer patients. The appearance of such tumor associated macrophages might have occurred already in chronic inflammation and early carcinogenesis as indicated by our recent study.4 However, a clear discrimination of M2-macrophages from M1-macrophages seems to be more applicable when analysing in vitro generated macrophages and should be avoided in analyses of inflammation associated colon cancer as i) resident lamina propria macrophages in IBD tissues exert an activated but anergic inflammatory phenotype rather than a fully immunosuppressive M2-phenotype, and ii) monocytes/macrophages are exposed to a continuously changing plethora of cytokines/growth factors generating a “mixed” phenotype with an excess of either pro- or anti-inflammatory effector functions. Hence, one can assume that during chronic inflammation macrophages differentially affect tumorigenesis as they change from a more pro- towards an anti-inflammatory phenotype (Fig. 1). Initially, pro-inflammatory macrophages prevail favouring the onset of carcinogenesis, e.g. by creating oxidative stress that induces adaptive responses of the stressed epithelium through activation of the transcription factor Nrf2.2 During the course of chronic inflammation, the microenvironment is increasingly influenced by anti-inflammatory macrophages that further sustain tumorigenesis, e.g. through TGF-β1 dependent EMT.4 However, besides the multitude of literature clearly demonstrating pro-tumorigenic functions of macrophages in CRC, some studies indicate anti-tumorigenic activities of macrophages being associated with better survival of CRC patients. These contradictory results may be explained by the fact that depending on the localization within the tumor and thereby on the environmental conditions (e.g. tumor margin versus hypoxic centre), macrophages may exhibit different effector functions.10 Taken together, a highly complex microenvironment created by macrophages exerting pro- and/or anti-inflammatory effects emerges early in colitis associated cancer developing along with (and concomitantly modulating) the onset and progression of CRC, yet further insights are needed to better understand this complexicity.
Figure 1.
Impact of pro- and anti-inflammatory macrophages on inflammation dependent initiation of tumorigenesis. During chronic inflammation, macrophages differentially affect tumorigenesis as they change from a pro- towards an anti-inflammatory phenotype. Initially, pro-inflammatory macrophages prevail and may favour the onset of carcinogenesis by inducing stress-adaptive responses of the inflammed epithelium (e.g. through Nrf2) as well as cellular survival and renewal (e.g. through STAT3, NF-κB). During the course of chronic inflammation, the microenvironment is increasingly influenced by anti-inflammatory macrophages that further sustain tumorigenesis, e.g. through TGF-β1 dependent EMT.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19949
References
- 1.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–16. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 2.Sebens S, Bauer I, Geismann C, Grage-Griebenow E, Ehlers S, Kruse ML, et al. Inflammatory macrophages induce Nrf2 transcription factor-dependent proteasome activity in colonic NCM460 cells and thereby confer anti-apoptotic protection. J Biol Chem. 2011;286:40911–21. doi: 10.1074/jbc.M111.274902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stadnicki A, Machnik G, Klimacka-Nawrot E, Wolanska-Karut A, Labuzek K. Transforming growth factor-beta1 and its receptors in patients with ulcerative colitis. Int Immunopharmacol. 2009;9:761–6. doi: 10.1016/j.intimp.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Schäfer H, Struck B, Feldmann EM, Bergmann F, Grage-Griebenow E, Geismann C, et al. TGF-β1-dependent L1CAM expression has an essential role in macrophage-induced apoptosis resistance and cell migration of human intestinal epithelial cells. Oncogene. 2012 doi: 10.1038/onc.2012.44. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Schäfer H, Geismann C, Heneweer C, Egberts JH, Korniienko O, Kiefel H, et al. Myofibroblast-induced tumorigenicity of pancreatic ductal epithelial cells is L1CAM dependent. Carcinogenesis. 2012;33:84–93. doi: 10.1093/carcin/bgr262. [DOI] [PubMed] [Google Scholar]
- 6.Gavert N, Conacci-Sorrell M, Gast D, Schneider A, Altevogt P, Brabletz T, et al. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol. 2005;168:633–42. doi: 10.1083/jcb.200408051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sebens Müerköster S, Werbing V, Sipos B, Debus MA, Witt M, Grossmann M, et al. Drug-induced expression of the cellular adhesion molecule L1CAM confers anti-apoptotic protection and chemoresistance in pancreatic ductal adenocarcinoma cells. Oncogene. 2007;26:2759–68. doi: 10.1038/sj.onc.1210076. [DOI] [PubMed] [Google Scholar]
- 8.Kaifi JT, Reichelt U, Quaas A, Schurr PG, Wachowiak R, Yekebas EF, et al. L1 is associated with micrometastatic spread and poor outcome in colorectal cancer. Mod Pathol. 2007;20:1183–90. doi: 10.1038/modpathol.3800955. [DOI] [PubMed] [Google Scholar]
- 9.Schäfer H, Dieckmann C, Korniienko O, Moldenhauer G, Kiefel H, Salnikov A, et al. Combined treatment of L1CAM antibodies and cytostatic drugs improve the therapeutic response of pancreatic and ovarian carcinoma. Cancer Lett. 2011 doi: 10.1016/j.canlet.2011.12.035. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Erreni M, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) and inflammation in colorectal cancer. Cancer Microenviron. 2011;4:141–54. doi: 10.1007/s12307-010-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]