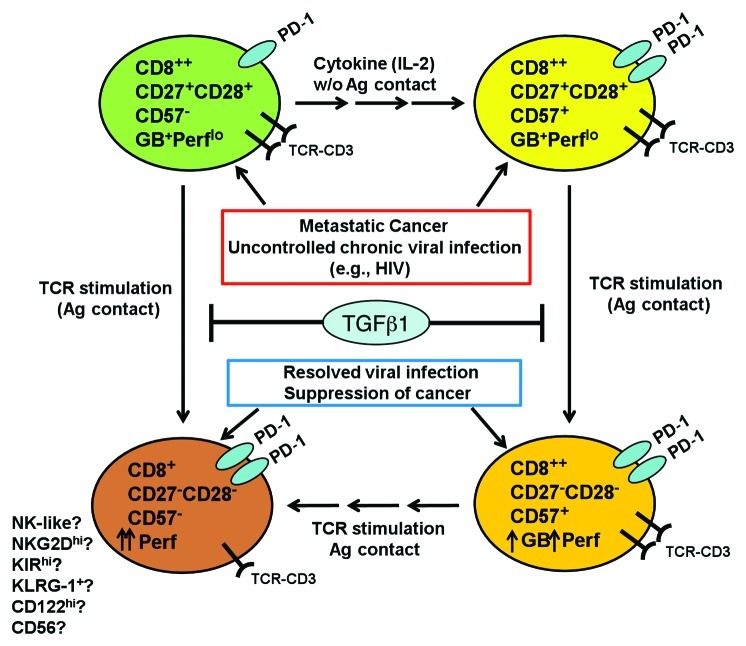
Figure 1. Differentiation pathway of the tumor-infiltrating CD8+ T cells in metastatic cancer. In situations where CD8+ T cells encounter persistent, chronic antigenic stimulation such as metastatic cancer or uncontrolled chronic viral infections, CD8+ effector-memory T (TEM) cells fail to coordinate downregulation of CD27 with upregulation of an end-stage CTL marker, CD57 and acquire a more cytolytic phenotype. Thus TEM fail to transition from a granzyme B (GB+) perforinlo (Perflo) cells into Perfhi, highly cytotoxic end-stage CTL. This resulted in accumulation of CD8+ T cells at a transitional stage where markers for early TEM (CD27, CD28) are co-expressed with CD57, even though the cells remain Perflo. We also found that TGF-β1, an immunosuppressive cytokine frequently found in the microenvironment of metastatic cancer, could also contribute to the arrested differentiation and accumulation of CD27+CD57- precursor T cells and CD27+CD57+ T cells. We found that PD-1 was expressed more in the CD27+CD57+ T cells, which implied that they may exhibit a higher level of effector activity. When these tumor-infiltrating lymphocytes (TIL) are expanded with IL-2, a minor fraction (~30%) of CD27+CD57- subset differentiated into CD27+CD57+ T cells. After TCR stimulation ex vivo, the CD27+CD28+CD57- subset directly differentiated to become CD27-CD57- T cells. On the other hand, CD27+CD57+ differentiated to become CD27-CD57+, and, in some patients, CD27-CD57-. These phenotypic changes were accompanied by increased perforin expression and acquisition of potent cytotoxicity against tumor cells. We think that CD57 is not a marker for T-cell senescence, but rather marks highly differentiated T cells that are in the process of transitioning into a truly end-stage, effector CTL. Currently it is not known which set of markers define truly senescent, end-stage, highly cytolytic CTL. We also propose that ultimately, both the CD8+CD57+ and CD8+CD57- subsets may ultimately differentiate into a subset of CD8+CD27-CD28-CD57- cells that may be more NK-like, expressing higher levels of CD122, KLRG1, NKG2D and other NK receptors.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.