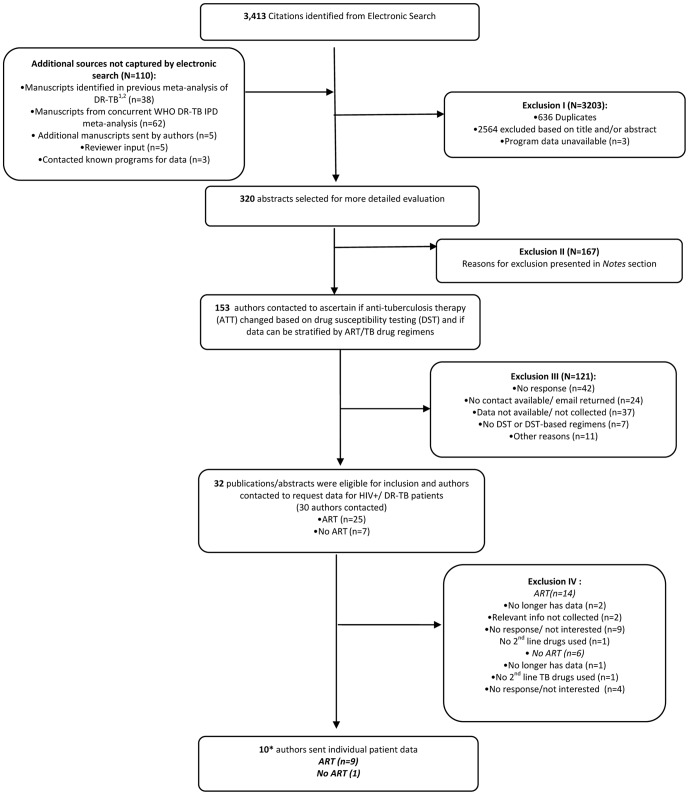
Figure 1. Flow diagram for study inclusion.
Two authors each sent data that was represented by two included studies, therefore 12 references actually included. 1 Lew W, Pai M, Oxlade O, Martin D and Menzies D. Initial drug resistance and tuberculosis treatment outcomes: systematic review and meta-analysis. Ann Intern Med 2008;149:123–34. 2 Menzies D, Benedetti A, Paydar A, et al. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Med 2009; 6:e1000150. Reasons for Exclusion II. Inappropriate study design (n = 34); Outcomes of interest are not measured (n = 22); Not deemed research/no data collected (n = 18); No TB drug resistance or drug resistance testing (n = 21); No HIV+ patients or HIV-testing (n = 37); No TB-infected patients or TB treatment (n = 4); No 2nd line drug TB therapy used (n = 16); No ART data collected (n = 3); Author contacted for another study/same patients (n = 12). Other Reasons for Exclusion III (other n = 11): Not interested (n = 1); Inappropriate study design (n = 1); No HIV (n = 3); In process of publication (n = 1); Already contacted (n = 5).