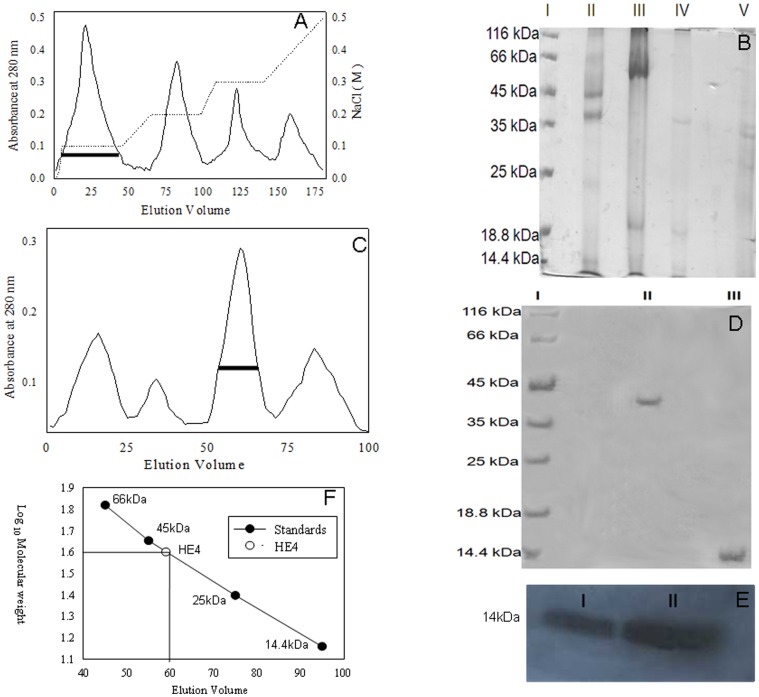
Figure 1. Purification of HE-4 protein from human seminal plasma using affinity, ion exchange and gel filtration chromatography.
(A) Elution profile of anion exchanger DEAE sephacel. The peaks formed as a function of X-axis as elution volume in ml and Y-axis is mA at 280 nm. The second (….) line represents the gradient of NaCl. The first peak with black base given is the one where we found HE-4. (B) 12.5% SDS-PAGE of peaks obtained from anion exchanger DEAE sephacel. Lane I: Protein marker. Lane II: Elution profile of First peak in which we obtained HE-4 with other impurities. Lane III, IV and V: Elution profile of second, third and fourh peak respectively. (C) Elution profile of Sephadex G-75. The peaks were obtained as a function of X axis as elution volume in mL and Yaxis is mA at 280 nm. Buffer used for elution is 50 mM Tris-HCl pH 8.0 and 0.2 M NaCl. (D) 12% SDS-PAGE of the third peak of elution profile of sephadex G-75. Lane I: Protein Marker. Lane II: Elution of the third peak in non-reducing condition and Lane III: Elution profile of a third peak in reducing conditions. (E) Immunodetection of HE-4 in crude seminal plasma and of the third peak of elution profile of sephadex G-75. Lane I: Crude seminal plasma. Lane II: Purified protein (IIIrd peak of sephadex G-75). (F) The standard curve for Sephadex G-75 and lines drawn for the position of HE-4 in the standard curve.