Abstract
A sensor to quickly and simply assess the relative reactivity of different hydrogen bonding catalysts has been developed. Specifically, blue shifts seen upon treatment of hydrogen bonding catalysts with the colorimetric compound, 7-methyl-2-phenylimidazo[1,2-a]pyrazin-3(7H)-one, correlate well to the Keq of binding to the sensor. The blue shifts also show a high degree of correlation with relative rates in Diels-Alder reactions of methyl vinyl ketone and cyclopentadiene employing the hydrogen bonding catalysts. The relevance of the sensor blue shifts to the LUMO lowering abilities of the hydrogen bonding catalysts is discussed.
Electrophile activation by small-molecule hydrogen bond donors has emerged as an important paradigm for enantioselective catalysis.i Nonetheless, a thorough understanding of the principles and features that govern the reactivity and selectivity of these catalysts remains incomplete. A number of physical organic measurements have provided scales that can be used to estimate the reactivity such as the pKa table,ii the nucleophilicity and electrophilicity parameters,iii the Irving – Williams orderiv,v etc but no scales have been made for all categories of H-bonding catalysts. Contributing to this problem is the large range of hydrogen bond strengths, from 0.2–40 kcal/mol.vi While the strength of a hydrogen bonding interaction can be inferred from ΔpKa,vii,viii such a measurement gives an incomplete account with respect to catalysis since a water molecule poorly mimics a substrate. As a result, secondary interactions, such as sterics, dual hydrogen bonding,ix and hydrogen bonding directionality, between a hydrogen bond donor and an electrophilic substrate are not fully incorporated. Here, we present a simple spectroscopic measurement using a colorimetric sensor to determine the effectiveness of hydrogen bonding catalysts in electrophilic activation of a monodentate substrate. The measurement is effective for a range of catalysts encompassing a pKa window of approximately 7–20.
We assessed a number of methods to judge the ability of different hydrogen bond donors to activate a carbonyl (LUMO lowering), but found that methods effective for strong Lewis acids, such as changes in IR or NMR signals, provided insufficient signal or were technically challenging. In search of a simple, easily applied measurement, we elected to use a colorimetric sensor molecule. 7-Methyl-2-phenylimidazo[1,2-a]pyrazin-3(7H)-one (1), which gives good correlations between λmax shifts and the Fukuzumi parameters for a small number of Lewis acidsx,xi,xii was discovered to give a readily discernable signal upon coordination (eq 1) with a range of hydrogen bond donors (Chart 1). Figure 1 illustrates the simplicity of the method with changes in color, which are readily visible to the naked eye, upon saturation with different hydrogen bonding catalysts.
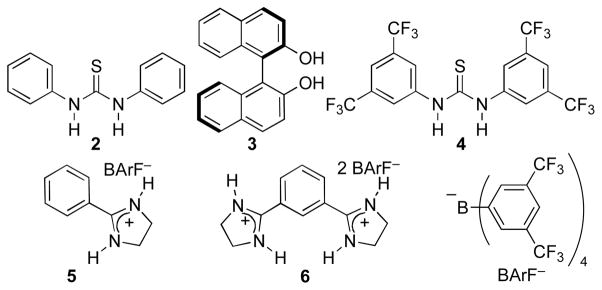
Chart 1.
Hydrogen bonding catalysts.
Figure 1.
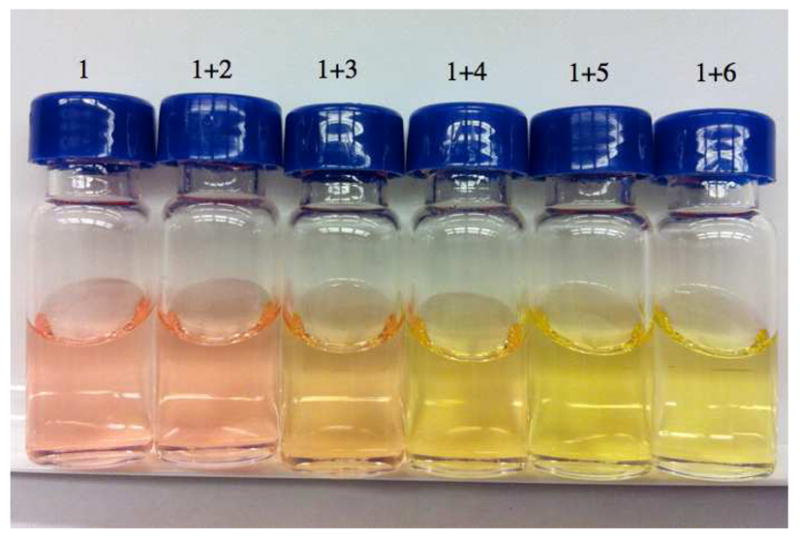
Change in color upon addition of hydrogen bonding catalysts (see Chart 1) to the pyrazinone sensor 1 in dichloromethane.
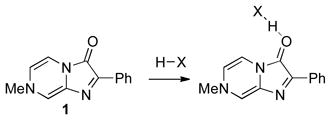 |
(1) |
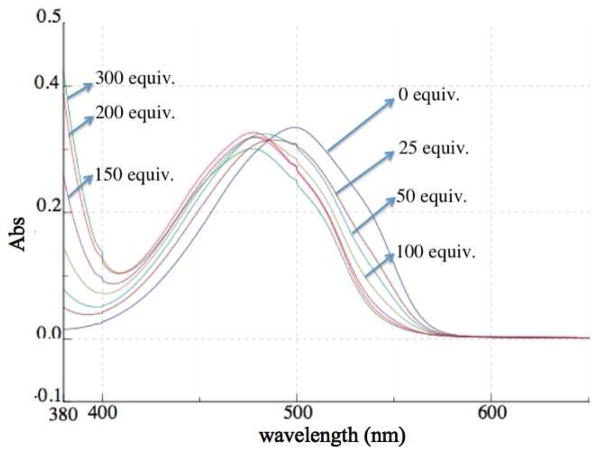
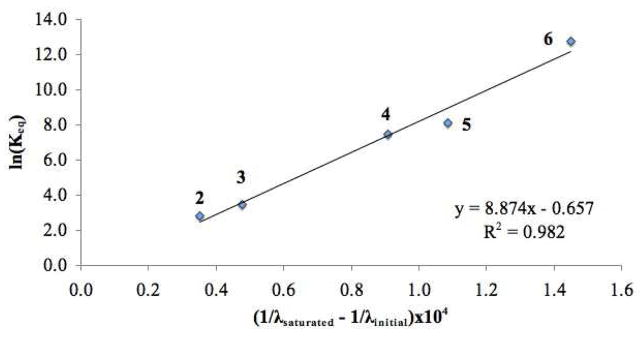
Figure 2 further illustrates the blue shift in the λmax of the sensor when combined with increasing amounts of a hydrogen bonding catalyst, in this case N,N′-di(3,5-bis(trifluoromethyl)phenyl)thiourea. With this data, Keq values (Table 1) for the sensor – hydrogen bond donor association xiii could be readily obtained from the corresponding titration curves as illustrated for N,N′-di(3,5-bis(trifluoromethyl)-phenyl)thiourea.xiv The inverse of the λmax shift obtained upon saturation with 2–6 showed a strong correlation with the Keq value (Figure 3) indicating that this λmax shift could be used as a reliable indicator of the association between the sensor and a prospective hydrogen bonding catalyst.
Figure 2.
Response of sensor 1 at 2.22×10−5 M to increasing amounts of N,N′-di(3,5-bis(trifluoromethyl)-phenyl)thiourea (4) in dichloromethane.
Table 1.
Hydrogen bonding catalyst saturated λmax values and Keq values for binding to 1 along with kcat values for the reaction in eq 2 at 1 mol% catalyst loading in benzene.
| Hydrogen bond donor | pKa (DMSO) | λmax (nm) | Keq (M−1) | kcat (s−1) |
|---|---|---|---|---|
| None | 499 | –a | ||
| 2 | 13.4vii | 490 | 1.67 × 10 | 1.26 × 10−6 |
| 3 | 17.1xv,b | 487 | 3.23 × 10 | 1.80 × 10−6 |
| 4 | 8.5vii | 477 | 1.77 × 103 | 2.09 × 10−5 |
| 5 | 12.8–13.6xvi | 473 | 3.34 × 103 | 4.90 × 10−5 |
| 6 | 12.8–13.6xvi,c | 465 | 3.47 × 105 | 1.79 × 10−4 |
kuncat = 7.50 ×10−5 s−1.
For 2-naphthol.
First pKa may be 1–2 units lower due to dicationic nature of 6.
Figure 3.
Correlation between wavelength shift and Keq.
Importantly, this sensor coordinates very weakly to water (Δλmax at saturation = 3.4 nm), which is easily displaced by catalyst. Thus, implementation is simple: sufficient catalyst is added until no further blue shift is seen. At this point, any water has been displaced and the sensor is saturated. The λmax obtained at this juncture is then used in the correlations to binding (Keq) and rate (krel, see below). For example, a measurement can be made using 10 μg of the sensor and ≤10 mg of the catalyst without special precautions to exclude moisture.
The Diels-Alder reactions of α, β-unsaturated carbonyl dienophiles is well established to undergo rate acceleration with Lewis acids by LUMO lowering of the dienophile xvii,xviii,xix,xx and a similar activation is believed to operate for hydrogen bonding catalysts.xxi In order to limit the number of different interactions between the substrates and the hydrogen bonding catalyst, the monodentate substrate methyl vinyl ketone was selected along with a nonbonding diene, cyclopentadiene (eq 2). Rate measurements by NMRxxii,xxiii showed a range of activities for different hydrogen bonding catalysts (Table 1).
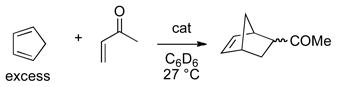 |
(2) |
A plot of ln(krel) (krel = kcat/kuncat) vs the inverse of the λmax shift (Figure 4) showed a strong correlation indicating that the binding to the sensor provides a reasonable account of the LUMO lowering ability of different hydrogen bonding catalysts. In contrast, the pKa values do not track well with the reactivity (Table 1, pKa vs kcat).
Figure 4.
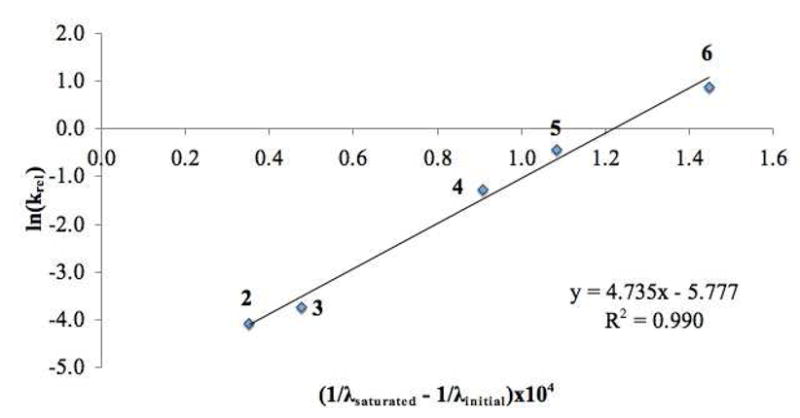
Correlation of Diels-Alder krel values from different hydrogen bonding catalysts with their wavelength shifts of sensor 1.
In conclusion, pyrazinone sensor 1 was found to rapidly provide a read out of the relative reactivity of hydrogen bonding catalysts in the Diels-Alder reaction of methyl vinyl ketone and cyclopentadiene. Namely, catalysts that cause a greater blue shift at saturation of the sensor are more reactive. Thus, it appears that the interaction between hydrogen bond donors and the carbonyl of the sensor provides a good approximation of the LUMO lowering potential available via hydrogen bonding. These preliminary results support the use of sensor 1 as a tool to gauge the relatively reactivity of new hydrogen bonding catalysts and to further the understanding of why different hydrogen bonding catalysts are more effective than others. Exploration of additional hydrogen bonding donors and Lewis acids with the pyrazinone sensor and with other reactions is underway.
Supplementary Material
Acknowledgments
Financial support was provided by the NIH (RO1GM087605). Partial instrumentation support was provided by the NIH for NMR (1S10RR022442). P. H. gratefully acknowledges the Vietnam Education Foundation for a fellowship.
Footnotes
Supporting Information. Experimental procedures, kinetics results, and spectral data. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- (i).For reviews, see: Taylor MS, Jacobsen EN. Angew Chem Int Ed. 2006;45:1520–1543. doi: 10.1002/anie.200503132.Doyle AG, Jacobsen EN. Chem Rev. 2007;107:5713–5743. doi: 10.1021/cr068373r.Connon SJ. Chem Eur J. 2006;12:5418–5427. doi: 10.1002/chem.200501076.Connon SJ. Angew Chem Int Ed. 2006;45:3909–3912. doi: 10.1002/anie.200600529.Connon SJ. Chem Commun. 2008:2499–2510. doi: 10.1039/b719249e.Pihko PM. Angew Chem Int Ed. 2004;43:2062–2064. doi: 10.1002/anie.200301732.Ting A, Schaus SE. Eur J Org Chem. 2007:5797–5949.Akiyama T, Itoh J, Fuchibe K. Adv Synth Catal. 2006;348:999–1010.Akiyama T. Chem Rev. 2007;107:5744–5758. doi: 10.1021/cr068374j.Nagasawa K, Sohtome Y. Synlett. 2010:000A–000V.Schenker S, Zamfir A, Freund M, Tsogoeva SB. Eur J Org Chem. 2011:2209–2222.Jensen KH, Sigman MS. J Org Chem. 2010;75:7194–7201. doi: 10.1021/jo1013806.
- (ii).Bordwell FG. Acc Chem Res. 1988;21:456–463. [Google Scholar]
- (iii).Mayr H, Kempf B, Ofial AR. Acc Chem Res. 2003;36:66–77. doi: 10.1021/ar020094c. [DOI] [PubMed] [Google Scholar]
- (iv).(a) Irving H, Williams RJP. Nature. 1948;162:746–747. [Google Scholar]; (b) Irving H, Williams RJP. J Chem Soc. 1953:3192–3210. [Google Scholar]
- (v).Examples of applications of Irving-Williams order: Evans DA, Rovis T, Johnson JS. Pure Appl Chem. 1999;71:1407–1415.Johnson JS, Evans DA. Acc Chem Res. 2000;33:325–335. doi: 10.1021/ar960062n.
- (vi).Steiner T. Angew Chem Int Ed. 2002;41:48–76. [Google Scholar]
- (vii).Jakab G, Tancon C, Zhang Z, Lippert KM, Schreiner PR. Org Lett. 2012;14:1724–1727. doi: 10.1021/ol300307c. [DOI] [PubMed] [Google Scholar]
- (viii).Gilli P, Pretto L, Bertolasi V, Gilli G. Acc Chem Res. 2009;42:33–44. doi: 10.1021/ar800001k. [DOI] [PubMed] [Google Scholar]
- (ix).Annamalai VR, Linton EC, Kozlowski MC. Org Lett. 2009;11:621–624. doi: 10.1021/ol8026945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (x).Takamuki Y, Maki S, Niwa H, Ikeda H, Hirano T. Tetrahedron. 2005;61:10073–10080. [Google Scholar]
- (xi).Fukuzumi S, Kei Ohkubo K. Chem Eur J. 2000;6:4532–4535. doi: 10.1002/1521-3765(20001215)6:24<4532::aid-chem4532>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- (xii).Fukuzumi S, Kei Ohkubo K. J Am Chem Soc. 2002;124:10270–10271. doi: 10.1021/ja026613o. [DOI] [PubMed] [Google Scholar]
- (xiii).Protonation of the pyrazinone sensor with methanesulfonic acid in DMSO has been reported (Nakai S, Yasui M, Nakazato M, Iwasaki F, Maki S, Niwa H, Ohashi M, Hirano T. Bull Chim Soc Jpn. 2003;76:2361–2387..) Based on the K value of protonation, the sensor can be determined to have a corresponding pK of -1.0 (DMSO). These data support interactions between the sensor and hydrogen-bond donors that do not involve proton tranfer.
- (xiv).See Supporting Information.
- (xv).Bordwell FG. Acc Chem Res. 1988;21:456–463. [Google Scholar]
- (xvi).Koppel I, Koppel J, Leito I, Grehn L. J Phys Org Chem. 1996;9:265–268. [Google Scholar]
- (xvii).Corey EJ, Loh T-P, Sarshar S, Azimioara M. Tetrahedron Lett. 1992;33:6945–6948. [Google Scholar]
- (xviii).Goering HL, Chang C. J Org Chem. 1975;11:2565. [Google Scholar]
- (xix).Alston PV, Ottenbrite RM. J Org Chem. 1975;8:1111–1116. [Google Scholar]
- (xx).Williamson KL, Hsu YL. J Am Chem Soc. 1970;92:7385–7389. [Google Scholar]
- (xxi).Huang Y, Unni AK, Thadani AN, Rawal VH. Nature. 2003;424:146. doi: 10.1038/424146a. [DOI] [PubMed] [Google Scholar]
- (xxii).Schreiner PR. Chem Soc Rev. 2003;32:289–296. doi: 10.1039/b107298f. [DOI] [PubMed] [Google Scholar]
- (xxiii).Wittkopp A, Schreiner PR. Chem Eur J. 2003;9:407–414. doi: 10.1002/chem.200390042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.