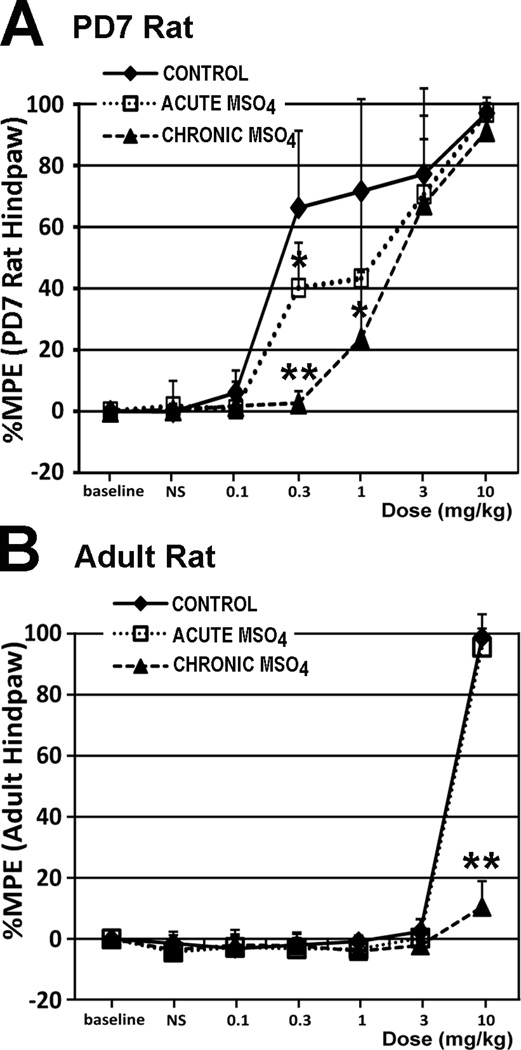
Figure 2. Behavioral Analysis of Morphine Antinociceptive Effects.
Hot Plate testing was done on the 7th day of treatment to evaluate development of antinociceptive tolerance (n=6/pharmacological group/age, except for n=7 for chronic morphine group in postnatal day (PD)7 rat). We used 49°C (with 20 s cutoff latency) for PD7 rats, and 56°C (with 12 s cutoff latency) for adult rats. Thus, morphine potency cannot be compared between two age groups. Chronic morphine treatment rendered both the the PD7 (F(2,16)=24.74; p<0.001 at 0.3 mg/kg testing dose) and adult rat (F(2,15)=228.9; p<0.001 at 10 mg/kg testing dose) tolerant to morphine’s antinociceptive effects. Results are presented as a percentage of maximum possible effect (%MPE ± SD) according to the method of Harris and Pierson (Harris and Pierson, 1964) to construct dose-response curves for morphine’s antinociceptive effect. Panel A: In the PD7 rat, a morphine dose of 0.3 mg/kg showed evidence of tolerance development in the chronic morphine group (%MPE= 2.67± 3.87), in comparison to both control (66.24% ± 25.08; p<0.01) and acute morphine (40.19% ± 14.68; p<0.01) groups. Interestingly, the same morphine dose of 0.3 mg/kg led to a statistically significant decrease in %MPE of the acute morphine group versus control group (p<0.05), suggesting that even a single prior exposure to morphine attenuates its antinociceptive effect at this early age. Furthermore, a 1 mg/kg morphine test dose led to a statistically significant difference (F(2,16)=5.17; p=0.01) only between the control and chronic morphine group (p<0.05). Panel B: In the adult rat, a morphine dose of 10 mg/kg led to significantly lower %MPE in the chronic morphine group (10.46% ± 8.45), in comparison to control (98.81% ± 2.91; p<0.01) and acute morphine (95.58% ± 10.8; p<0.01) groups. Abbreviations: MSO4, morphine; **, p<0.01; *, p<0.05.