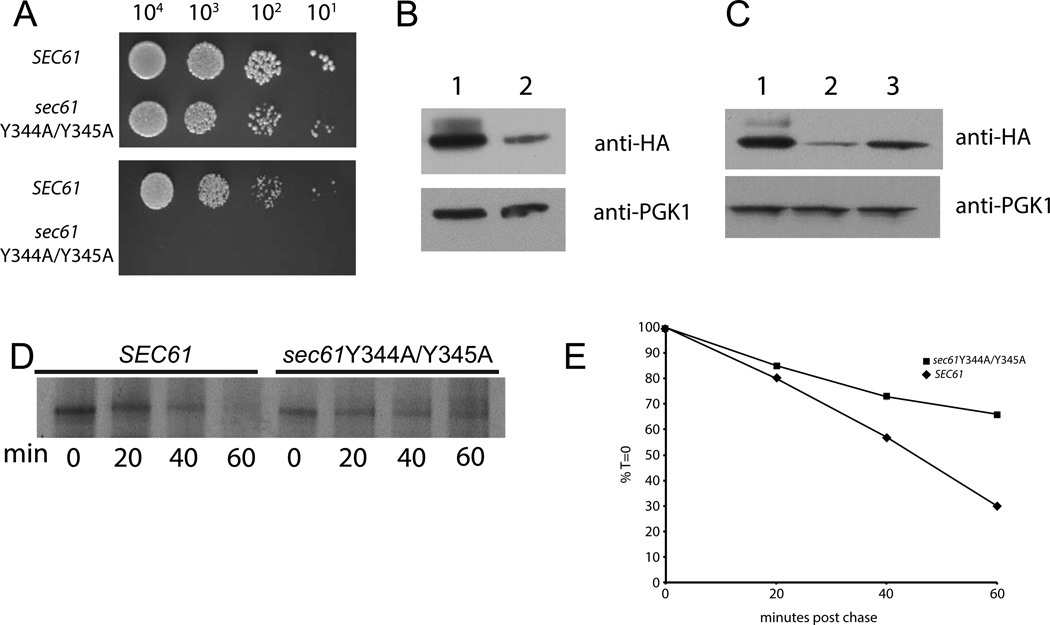
Figure 4. Tyrosines 344 and 345 are necessary for stability of the Sec61 protein.
Yeast strains were made as in Figure 1, except that sec61Y344A/Y345A was used instead of sec61Y345H. (A) Yeast of the indicated genotype were grown at 30° to an OD of 0.8 and plated at the indicated concentrations on plates containing either no stressor (top) or 500 ng/mL tunicamycin (bottom) and incubated at 30°. (B) Lysates from either SEC61 (lane 1) or sec61Y344A/Y345A (lane 2) yeast were fractionated by SDS-PAGE and transferred to PVDF membrane and probed with an anti-HA antibody; the same membrane was stripped and then re-probed with anti PGK1 antibody which serves as a loading control. (C) SEC61 (lane 1), sec61Y344A/Y345A (lane 2), sec61Y344A/Y345A gal-SSS1 (lane3) yeast were grown at 30° in media with 2% galactose as a carbon source to induce expression of Sss1p. Lysates were prepared and treated as in B. For CPY* degradation (D) yeast expressing CPY*-FLAG, gal-SSS1 and the indicated Sec61 allele growing in log phase in galactose-containing medium were harvested and labeled with 35S-cysteine/methionine and chased with cold media for the indicated time points. Yeast were then lysed, immunoprecipitated with anti-FLAG antibody and fractionated by SDS-PAGE. A representative experiment is shown. (E) Quantitation of the experiment shown in D.