Abstract
Rapamycin, a potent immunomodulatory drug, has shown promise in the amelioration of numerous age-associated diseases including cancer, Alzheimer’s disease and cardiac hypertrophy. Yet the elderly, the population most likely to receive therapeutic rapamycin, are already at increased risk for infectious disease; thus concern exists that rapamycin may exacerbate age-associated immune dysfunctions and worsen infection outcomes. Herein, we examined the impact of enteric delivered rapamycin monotherapy (eRapa) on susceptibility of aged (22–24 month) C57BL/6 mice to Streptococcus pneumoniae, the leading bacterial cause of community-acquired pneumonia. Following challenge with S. pneumoniae, administration of eRapa conferred modest protection against mortality. Reduced mortality was the result of diminished lung damage rather than reduced bacterial burden. eRapa had no effect on basal levels of Interleukin (IL)-1α, IL-6, IL-10, IL-12p70, KC, Interferon-γ, Tumor necrosis factor α and Monocyte chemotactic protein-1 in whole lung homogenates or during pneumococcal pneumonia. Previously we have demonstrated that cellular senescence enhances permissiveness for bacterial pneumonia through increased expression of the bacterial ligands Laminin receptor (LR), Platelet-activating factor receptor (PAFr) and Cytokeratin 10 (K10). These proteins are co-opted by S. pneumoniae and other respiratory tract pathogens for host cell attachment during lung infection. UM-HET3 mice on eRapa had reduced lung cellular senescence as determined by levels of the senescence markers p21 and pRB, but not mH2A.1. Mice on eRapa also had marked reductions in PAFr, LR, and K10. We conclude that eRapa protected aged mice against pneumonia through reduced lung cellular senescence, which in turn, lowered bacterial ligand expression.
Keywords: aging, rapamycin, pneumonia, cellular senescence, Streptococcus pneumoniae
1. INTRODUCTION
Rapamycin, an mTOR Complex 1 inhibitor, when fed to mice via enteric-coated microcapsules in rodent chow, substantially extends lifespan (Harrison et al., 2009; Miller et al., 2011). In addition to this, rapamycin has shown promise in the amelioration of cognitive deficits in mouse models of Alzheimer’s disease (Caccamo et al., 2010; Spilman et al., 2010), reduced the formation of protein inclusions found in numerous neurodegenerative diseases (Caccamo et al., 2009), prevented cardiac hypertrophy (Finckenberg and Mervaala 2010), and has well established anti-tumor properties (Albert et al., 2010). Less recognized, rapamycin has also been shown to prevent development of cellular senescence (Demidenko and Blagosklonny 2008; Demidenko et al., 2009; Leontieva and Blagosklonny 2010), a phenomenon whereby cells with shortened telomeres or those that have undergone DNA damage (i.e., genotoxic stress) lose the capacity to replicate without undergoing apoptosis and adopt a pro-inflammatory secretory phenotype (Campisi and d’Adda di Fagagna 2007). Thus, rapamycin has manifold benefits for a wide range of age-associated conditions and diseases.
Pneumonia, due both to viral and bacterial infection, is the fourth leading cause of death for those >65 years of age (Lopez et al., 2006; Minino et al., 2006). Numerous age-related changes in lung function, a decrease in innate and adaptive immunity, and the presence of age-associated co-morbidities such as cardiovascular disease, collectively contribute to the enhanced occurrence and severity of pneumonia among the elderly (Lexau et al., 2005). Most recently, we have demonstrated that senescent cells, which accumulate within the lungs and other aged tissues (Kreiling et al., 2011), enhance permissiveness for bacterial pneumonia through increased expression of inflammation- (i.e. Nuclear-factor kappa B [NFκB]-) and Retinoblastoma protein (pRB)-regulated proteins that pathogenic bacteria co-opt for lung cell adhesion and blood stream invasion. Briefly, this includes Laminin Receptor (LR), Platelet activating factor receptor (PAFr), and Cytokeratin 10 (K10); which in turn are bound to by Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus among other bacterial pathogens (Shivshankar et al., 2011). Thus, the individuals that might benefit the most from therapeutic rapamycin, the elderly, are also at increased risk for bacterial pneumonia due in part to senescence-associated changes in lung protein expression.
Given rapamycin’s well-established immunomodulatory properties, which include altered T-cell development and dampened antigen presenting cell function (Araki et al., 2009; Battaglia et al., 2005; Hackstein et al., 2003), enthusiasm for the drugs potential as a broad range therapeutic has thus far been modest. Concerns about immunosuppressive consequences of rapamycin are supported by considerable anecdotal evidence for opportunistic infections in transplant recipients receiving rapamycin to prevent organ rejection (Groth et al., 1999; Husain et al., 2003; Webster et al., 2006). Nonetheless, the effect of prolonged rapamycin monotherapy on the ability of otherwise healthy aged animals to resist pneumonia is not known. Furthermore, as rapamycin has been reported to inhibit cellular senescence, it may instead protect aged animals through reduced bacterial ligand expression in the lungs. Thus, the impact of rapamycin on bacterial pneumonia remains an open question.
Herein, we examined the impact of enteric delivered rapamycin monotherapy (eRapa) on susceptibility of aged mice to Streptococcus pneumoniae (the pneumococcus), a Gram-positive bacterium and the leading bacterial cause of community-acquired pneumonia (Johansson et al., 2010). We tested the impact of eRapa on disease severity, the acute pro-inflammatory cytokine response, cellular senescence in the lungs, and bacterial ligand expression. Against expectation, we show that eRapa conferred modest protection against death; possibly through reduced cellular senescence and bacterial ligand expression.
2. MATERIALS AND METHODS
2.1 Mice
For experiments that examined senescent markers and bacterial ligand levels within the lungs, UM-HET3 mice, the progeny of CB6F1 mothers and C3D2F1 fathers, were used. These mice, housed at The University of Michigan, received rodent chow ad libitum containing polymer-encapsulated rapamycin (i.e. eRapa) at 4.7- (low-), 14- (mid-), or 42-ppm (high-dose) rapamycin for 13 months from 9 to 22 months of age. Control mice received normal chow. For experiments involving bacterial challenge, male and female C57BL/6 mice were purchased from The Jackson Laboratories (Bar Harbor, Maine) and housed at The University of Texas Health Science Center. Mice were fed chow containing 14-ppm rapamycin such that they received 2.2 mg of rapamycin/kg body weight per day. This is the same dose and drug delivery formulation previously found to be neuroprotective and life extending (Caccamo et al., 2009; Caccamo et al., 2010; Harrison et al., 2009). Control mice received chow containing an equivalent amount of the encapsulating agent (Eudragit® Darmstadt, Germany) alone (Harrison et al., 2009). From 4 months of age C57Bl/6 mice received either eRapa long-term for 20 months (LT-eRapa), the control diet for 20 months (control), or the control diet for 16 months then eRapa for 4 months (ST-eRapa). For both sets of mice, activity of rapamycin was confirmed periodically by testing for phosphorylation of ribosomal protein S6 (at Ser 240 and Ser 244), a substrate of S6 kinase 1, in visceral adipose tissue of sentinel mice that received the diet (data not shown) (Miller et al., 2011). All mice were housed in specific pathogen free conditions.
2.2 Immunoblotting
Whole lung homogenates were prepared in lysis buffer and protein concentrations were determined via bicinchoninic acid assay. For each biological sample, 30 μg of sample was separated by SDS-PAGE under reducing conditions and proteins transferred onto nitrocellulose using a Semi-Dry Electrophoretic system (Bio-Rad, Hercules CA). Antibodies targeting PAFr (Cat# 160602, Cayman Chemical Co., Ann Arbor, MI), LR (Cat# MS-259-PIABX, NeoMarkers, Fremont, CA), K10 (Cat# Ab9026, Abcam, Cambridge, MA), pRB (Cat# 9309, Cell Signaling, Danvers, MA), p21 (Cat# SC-6246, Santa Cruz Antibody, Santa Cruz, CA), and histone macro H2A.1 (mH2A; Cat# 07-219, Millipore, Billerica, MA) were all obtained from commercial sources and incubated according to the manufacturers’ specifications. Membranes were subsequently probed using horseradish peroxidase conjugated secondary antibodies at 1:10,000 dilution using standard chemiluminescent methods. To confirm protein load, membranes were stripped and probed with antibody against actin (Cat# A300-48485A, Bethyl Laboratories, Montgomery, TX). Band intensities on autoradiographs were determined using Quantity One software (Bio-Rad).
2.3 Pathogen challenge and assessment of disease severity
Mice were challenged by forced inhalation of S. pneumoniae serotype 4 strain TIGR4 in 100 μl saline (Orihuela et al., 2004; Tettelin et al., 2001). Aged mice received 1.0 × 103 cfu for mortality and subsequent pathological and lung burden studies. All cohorts were divided equally between male and female mice and the infectious dose administered was verified. Disease severity was assessed by survival over time, quantitation of bacterial burden in the lungs and blood at fixed time points, and pathological examination of paraffin-embedded lung sections (Shivshankar et al., 2011). For pathological examination, two non-adjacent lung sections from each mouse were Hematoxylin and Eosin (H&E) stained and examined microscopically. These were scored blindly on a scale of 0–5 on the basis of peribronchial and perivascular inflammation, neutrophil infiltration, and alveolar consolidation. Levels of [Interleukin (IL)-6, IL-10, IL-12p70, Interferon (IFN)-γ, tumor necrosis factor (TNF)-α] and [Monocyte chemotactic protein (MCP)-1] in whole lung homogenates of the right inferior lobe were determined using a BD™ Cytometric Bead Array (CBA) - Mouse Inflammation Kit (BD Biosciences, San Jose, CA). Levels of IL-1α and KC were determined using mouse ELISA kits also from BD Biosciences.
2.4 Statistical analyses
Statistical analysis on survival following pathogen challenge was performed using a Kaplan-Meier Log Rank Test using the Holm-Sidak method. A One-Way ANOVA was used to compare bacterial titers, cytokine levels, and densitometric immunoblot values from eRapa fed groups versus the control for aged mice.
3. THEORY
Herein, we evaluated whether monotherapy with eRapa altered the susceptibility of aged mice to Streptococcus pneumoniae. Briefly, S. pneumoniae is a Gram-positive bacterium and a leading cause of community-acquired pneumonia (Johansson et al., 2010).
4. RESULTS
4.1 Rapamycin reduces pre-infection levels of pneumococcal ligands and senescent markers
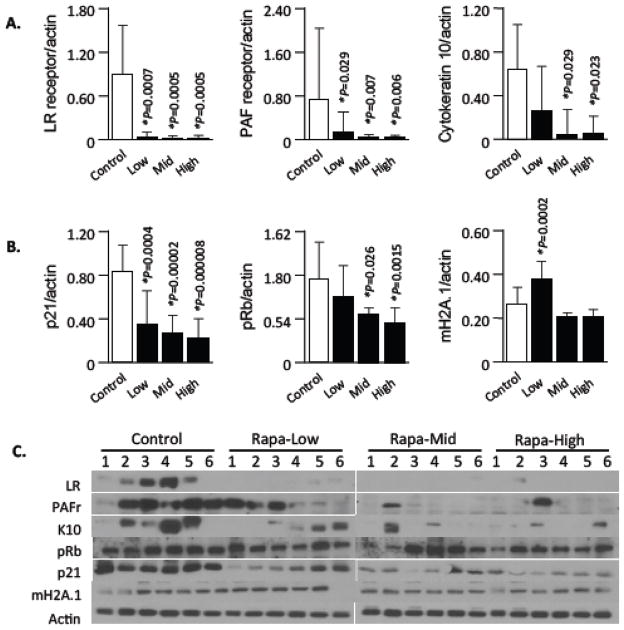
Elevated lung levels of host proteins that are co-opted by respiratory tract pathogens for attachment and cell invasion are one reason for the enhanced susceptibility of the elderly to pneumonia (Hinojosa et al., 2009). Previously, we have shown that cellular senescence contributes to this phenomena through increased levels of LR, PAFr, and K10 on lung epithelial cells (Shivshankar et al., 2011). Strikingly, we observed that healthy aged UM-HET3 mice receiving low-, mid-, and high-dose eRapa for 13 months had dramatic and significant reductions in basal lung levels of all three ligands versus the controls (Fig 1A, C). Moreover, we observed a strong reduction in levels of the senescent marker p21, a cyclin-dependent kinase inhibitor that checks the progression of the cell cycle at G1 (Child and Mann 2006), and to a lesser extent a dose-dependent reduction for retinoblastoma protein (pRB), a second senescent marker and tumor suppressor protein that binds and inhibits transcription factors of the E2F family (Fig 1B, C) (Manning and Dyson 2011). In contrast, we observed a modest but significant increase for mH2A.1, a third senescence marker, for mice on the low-eRapa diet. mH2A.1 is an indicator of accumulated DNA damage/heterochromatin complexes within the nuclei (Kreiling et al., 2011). Nonetheless, no changes were apparent for mH2A.1 in mice receiving the mid- or high-eRapa diet (Fig 1). Thus, eRapa was associated with a sharp reduction in bacterial ligand expression that has previously been shown to enhance permissiveness for bacterial attachment to lung cells and was concomitant with decreased levels of two well-established senescence associated proteins.
Figure 1. Effects of eRapa diet on the expression of pneumococcal ligands and senescence markers in the lungs.
Representative immunoblots and densitometric analysis of pneumococcal ligands and senescence markers in tissue lysates prepared from lungs of 22 month UM-HET3 mice that had received low-, mid-, and high-dose eRapa or control diet for 13 months (n=6/cohort). Antibodies used are displayed on the left. Actin was probed on the same membranes and used as a loading control. Histograms reveal the ratio (protein/actin) intensity values with error bars demonstrating standard errors of the mean. Statistical analysis was performed using a one-way ANOVA comparing the rapamycin fed groups versus the control diet. Asterisk denotes a statistically significant difference (P<0.05). Error bars show standard errors of the mean.
4.2 Effect of rapamycin monotherapy on mortality and pneumococcal burden
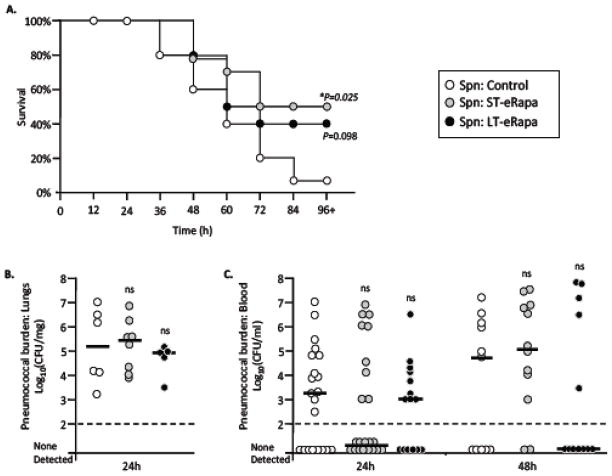
We infected aged C57BL/6 mice on short-term (ST) and long-term (LT) eRapa to determine its impact on severity of pneumococcal pneumonia. Figure 2 outlines the administered eRapa diets, which contained rapamycin at the dose previously documented to extend lifespan (Harrison et al., 2009). Aged mice on ST-eRapa had improved survival versus controls following intratracheal challenge with S. pneumoniae strain TIGR4 (ST-eRapa n=14, control n=15, P=0.025). A non-significant but similar positive trend was observed for aged mice on LT-eRapa (n=10, P=0.098) (Fig 3A). Of note, no differences in bacterial titers were observed in the lungs of aged mice after 24 hours versus control (Fig 3B). Nor was there a difference in the blood at 24 and 48 hours post infection when comparing aged ST-eRapa or LT-eRapa to control mice (Fig 3C).
Figure 2. Dietary regimens used for bacterial challenge assays.
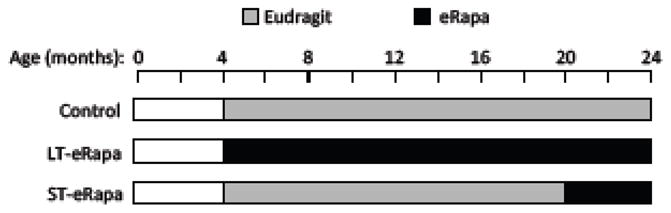
From 4 months of age, C57BL/6 mice were fed rodent chow containing rapamycin encased in Eudragit® (eRapa) or chow containing the encapsulating agent alone (Eudragit). Aged mice received either the control diet for 20 months (control), eRapa for 20 months (LT-eRapa), or the control diet for 16 months then eRapa for 4 months (ST-eRapa).
Figure 3. Effect of eRapa on survival and bacterial burden following pneumococcal challenge.
A) Kaplan Meier plot illustrating the survival over time of aged (24 month) C57BL/6 mice that had been fed rapamycin short-term (ST-eRapa; n=14), long-term (LT-eRapa; n=10), or the Eudragit (Control; n=15) diet following intratracheal challenge with 1.0 × 103 cfu of S. pneumoniae serotype 4 strain TIGR4. Statistical analysis was performed using a Kaplan-Meier Log Rank Test using the Holm-Sidak method. Asterisk denotes a statistically significant difference (P<0.05). Bacterial burden in the B) lungs and C) blood was assessed per gram of isolated lung tissue and volume of blood, respectively. For each experimental cohort, values for individual mice are represented as circles whereas the horizontal bar indicates the median value for the cohort. Statistical analysis was performed using one-way ANOVA comparing eRapa groups versus the control. Experimental cohort size for bacterial burden was as follows: For lungs, Control n=6, ST-eRapa n=8, LT-eRapa n=5; for blood, Control n=21, ST-eRapa n=22, LT-eRapa n=15.
4.3 Impact of rapamycin monotherapy on lung pathology
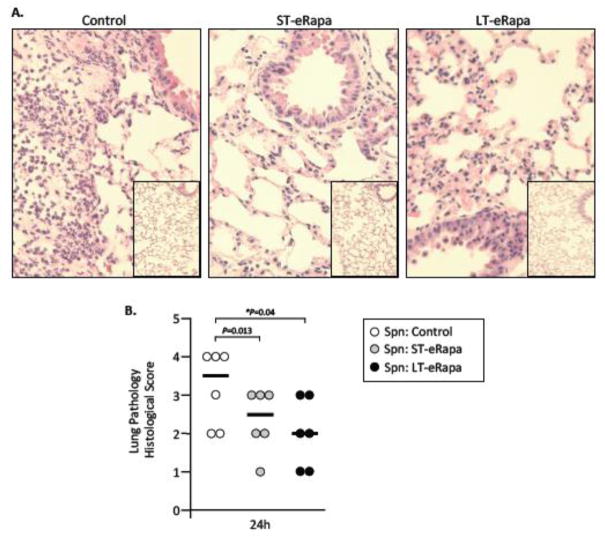
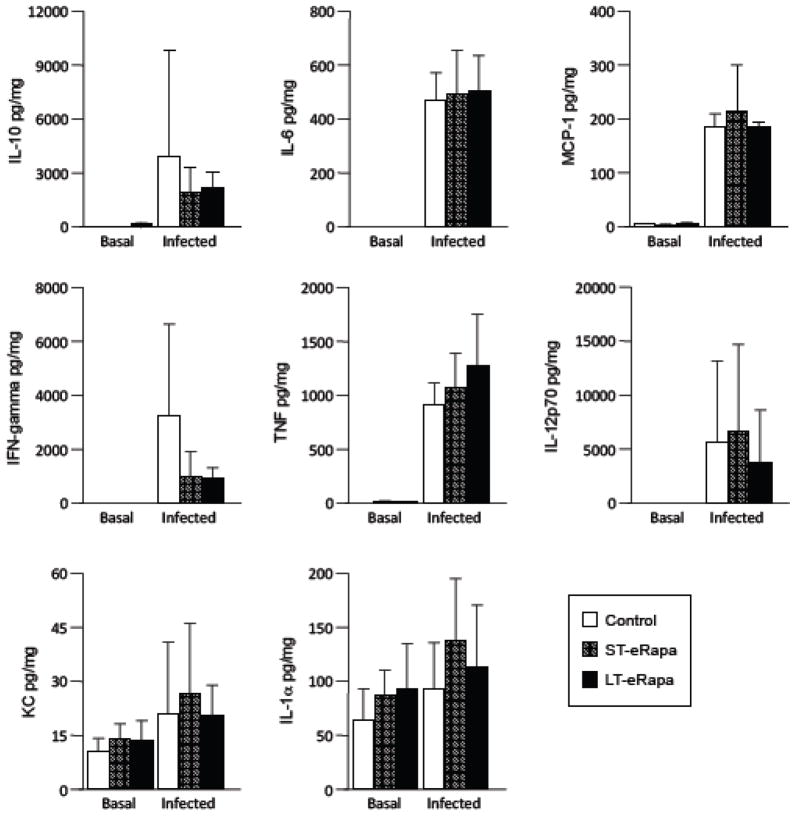
Consistent with the delayed mortality observed for aged mice on eRapa, pathological examination of H&E stained lung sections from infected mice after 24 hours revealed that infected C57BL/6 mice on ST-eRapa had a significantly reduced peribronchial and perivascular inflammation, decreased immune-cell infiltration, and less alveolar thickening than mice on the Eudragit controls, whereas mice on LT-eRapa trended towards the same (P=0.13) (Fig 4). No obvious differences in lung pathology were apparent in the sham-infected controls or between ST-eRapa or LT-eRapa infected mice. Given that rapamycin and inhibition of mTOR Complex 1 has been shown to inhibit de novo cytokine production by antigen presenting cells (Hackstein et al., 2003), we also examined whole lung homogenates for eRapa-induced alterations in the acute pro-inflammatory cytokine (i.e. IL-1α, IL-6, IL-12p70, IFN-γ, and TNFα), anti-inflammatory cytokine (i.e. IL-10), and pro-inflammatory chemokine (i.e. KC, MCP-1) response. No physiologically relevant differences were observed for mice on eRapa (ST-eRapa n=8, LT-eRapa n=5) in the production of these cytokines versus controls (n=6) during infection, nor were differences in basal levels of these inflammatory molecules observed in lung samples collected from uninfected aged C57BL/6 mice (control n=3, ST-Rapa n=5, LT-eRapa n=5) (Fig 5). Thus, eRapa did not alter the resting or acute cytokine response during infection, yet was associated with decreased tissue damage and vascular leakage, and reduced mortality.
Figure 4. Impact of eRapa on lung pathology.
A) Representative micrographs of H&E stained lung sections from infected and mock-infected aged mice on control, ST- and LT-eRapa diets 24 hours post-intratracheal challenge with S. pneumoniae (n=6 per cohort; infectious dose 1.0 × 104 cfu). Micrograph of mock-infected mice are inset within the larger image from infected mice. B) Lung histopathological scores for each infected mouse (individual circles) at 24 hours post-infection. Lungs were scored 0–5 on the basis of peribronchial and perivascular inflammation, neutrophil infiltration, and alveolar consolidation. For each mouse two separate non-adjacent lung sections were examined. The horizontal bar indicates the median score for the cohort. Statistical analysis was performed using one-way ANOVA comparing eRapa groups versus the control.
Figure 5. Cytokine and chemokine analysis in the lungs of uninfected and infected mice.
Basal and post-infection acute cytokine and chemokine profile in lungs from aged (24 month) C57BL/6 mice that had been fed rapamycin short-term (ST-eRapa: basal n=5, acute n=8), long-term (LT-eRapa: basal n=5, acute n=5), or the Eudragit (Control: basal n=3, acute n=6) diet. Mice were challenged intratracheally with 1.0 × 103 cfu of S. pneumoniae serotype 4 strain TIGR4, lungs were collected and homogenized for cytokine/chemokine analysis. Statistical analysis was performed using a one-way ANOVA comparing the rapamycin fed groups versus the control diet. Asterisk denotes a statistically significant difference (P<0.05).
DISCUSSION
While rapamycin and possibly other mTOR Complex I inhibitors, hold considerable promise as therapeutic agents to treat or delay the onset of Alzheimer’s disease and other age-associated diseases (Caccamo et al., 2009; Caccamo et al., 2010), there is a valid concern that the health benefits observed for rapamycin might be offset in the pathogen-rich real world by enhanced susceptibility to infectious diseases. This issue is particularly pressing for the elderly, the patient population most likely to use and benefit from rapamycin, as they are already at increased risk for pneumonia (Lexau et al., 2005; Lopez et al., 2006). As such, studies similar to this one, with aged animals on prolonged rapamycin monotherapy, are necessary to examine the impact of immune modulatory drugs on susceptibility to infectious diseases. One important caveat to this study is that the analyses of senescent markers and bacterial ligands were done using lung samples from heterogeneous UM-HET3 mice, whereas the susceptibility to pneumococcal pneumonia studies were done using inbred C57BL/6 mice. Thus, and despite the fact that rapamycin reduced cellular senescence and pneumococcal ligand expression in the lungs of genetically diverse animals, it is possible that the protection conferred by eRapa against pneumonia in the C57BL/6 mice was for other reasons and caution should be taken in the interpretation of our results.
We have previously demonstrated that cellular senescence results in the increased expression of bacterial ligands that respiratory tract pathogens co-opt for lung infection (Shivshankar et al., 2011). LR serves as a ligand for S. pneumoniae, H. influenzae and Neisseria meningitidis (Orihuela et al., 2009). K10 is a ligand for S. pneumoniae and S. aureus (O’Brien et al., 2002; Shivshankar et al., 2009). PAFr serves as a ligand for S. pneumoniae, H. influenzae, and Pseudomonas aeruginosa as well as many other bacteria that have surface-exposed phosphorylcholine (Barbier et al., 2008; Cundell et al., 1995; Swords et al., 2000). Respiratory tract pathogens also bind to other NFkB-regulated proteins such as intercellular adhesion molecule 1 (ICAM-1) and carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) that were not examined, but are also most likely up regulated as a result of the pro-inflammatory phenotype of senescent cells (Avadhanula et al., 2006; Conners et al., 2008). Thus, the decreased expression of bacterial ligands in the lungs of mice treated with eRapa suggests mTOR inhibition as a potential mechanism to decrease susceptibility to a broad range of bacterial pathogens. What is more, the concomitant reduction in senescent markers along with pneumococcal ligands in the lungs reinforces the notion that cellular senescence directly contributes to the susceptibility of the elderly to pneumonia.
Rapamycin’s broad effects are due to the fact that mTOR is a key component of multiple and overlapping cell-signaling pathways including those that affect de novo protein production, autophagy, T-cell differentiation, growth, and cell proliferation. Indeed, rapamycin has been demonstrated to have a potent anti-inflammatory effect due to its selective expansion of immunosuppressive T-regulatory cells (Araki et al., 2009; Battaglia et al., 2005), and its ability reduce cytokine production by antigen presenting cells following stimulation (Hackstein et al., 2003). It is for these reasons, the Food and Drug Administration has approved rapamycin along with calcineruin inhibitors to prevent organ rejection following transplantation. Unexpectedly, we observed no effect of prolonged low-dose enteric rapamycin delivery on basal cytokine levels or the ability of mice to respond to an infection despite the fact that eRapa suppressed LR and PAFr protein levels, which are encoded by NFkB-regulated genes. Reasons for the discrepancy in cytokine production between our study and others may include the strain of mouse tested, dose and duration of rapamycin, and route of drug administration. Importantly, it remains unclear at the molecular level how rapamycin suppresses pneumococcal ligand expression or that of the senescent markers in the lungs.
Several studies by Blagosklonny and colleagues have examined the effect of rapamycin on cellular senescence (Demidenko and Blagosklonny 2008; Demidenko et al., 2009; Leontieva and Blagosklonny 2010). These studies demonstrated that inhibition of mTOR in vitro either by serum starvation or rapamycin had a protective effect against stress-induced senescence. Blagosklonny and colleagues also showed that DNA damaging agents did not cause senescence in quiescent cells, while they do so in cells where mTOR is active (Leontieva and Blagosklonny 2010). Our experimental results support these observations and indicate that rapamycin reduces cellular senescence in vivo as measured by pRB and p21 levels. These markers are elevated in aged mice and were detectable in the control diet mice most likely as a result of normal aging processes and the accumulation of senescent cells.
Importantly, the findings published by Blagosklonny and colleagues does not suggest that rapamycin protect cells against DNA damage, but instead that rapamycin (i.e. mTOR inhibition) prevents activation of the cell-signaling pathways that leads to permanent cell cycle arrest. Along this line, we speculate that eRapa does not inhibit the damage or the signals that are responsible for enhanced NFkB-mediated pneumococcal ligand expression or pRB-mediated K10 expression but instead dampens the cellular response to these signals; possibly at the translational level. In support of this notion, we did not observe a decrease in the senescence marker mH2A.1, which is associated with heterochromatin remodeling following DNA damage (Kreiling et al., 2011).
Pneumococcal disease is characterized by intense inflammation at the affected site. Excessive inflammation results in vascular leakage, alveolar consolidation, and a loss of gas-exchange capability that often leads to death (Orihuela and Tuomanen 2006). Indeed this process was observed in all the experimentally infected mice and could be correlated with the overall mortality rates for the eRapa and control cohorts. Surprisingly, despite reduced lung pathology, which can in part be explained by reduced bacterial adhesion and invasion, we observed no differences in cytokine levels for the ST-eRapa or LT-eRapa cohorts versus controls. One possible explanation is that rapamycin promotes lung cell apoptosis over necrosis, the former that is immunoquiescent and anti-inflammatory in comparison to necrosis (Zhang et al., 2010). For example, rapamycin has been demonstrated to induce apoptosis in tumor cells by reducing the expression of Bcl-XL an inhibitor of apoptosis (Tirado et al., 2005). Rapamycin also expands the numbers of CD4+CD25+Foxp3+ T-regulatory cells (Battaglia et al., 2005). T-regulatory cells are present in humans with acute lung injury and have been shown to help resolve experimental lung injury in mice (D’Alessio et al., 2009). Importantly, we only tested a small number of cytokine/chemokine/immune modulators. Further experimentation is required to resolve this speculation and assess the impact of eRapa on apoptosis and T-regulatory cells following cellular insult.
Our study is not the first to examine the effects of rapamycin on infection, although it is the first to test the effects of prolonged monotherapy. Recently, Fielhaber et al. showed that intraperitoneal administration of rapamycin at 1.5 mg/kg six hours before challenge with lipopolysaccharide enhanced lung injury. This occurred as a result of inhibited LPS-induced apoptosis, yet was associated with reduced NFkB activation, reduced neutrophil infiltration, and diminished pro-inflammatory cytokine production (Fielhaber et al., 2012). In addition to differences in dose and drug schedule, our study is different in that S. pneumoniae is a Gram-positive bacterium and thereby lacks lipopolysaccharide, a highly potent pro-inflammatory and cytotoxic molecule. Other investigators report that rapamycin induced changes in autophagy following influenza infection that enhanced apoptosis within infected cells and worsened tissue damage (Sun et al., 2012). In contrast, rapamycin inhibited the development of Chlamydia pneumoniae, an obligate intracellular pathogen (Yan et al., 2010). Thus the impact of rapamycin on susceptibility to infection most likely depends on a wide range of factors that includes Gram-positive versus Gram-negative, intracellular versus extracellular, and host mechanisms of clearance. Importantly, these etiological agents must also be tested in context of an aged mouse in order to have some clinical applicability. Finally, the impact of short versus long-term rapamycin administration is also an important issue, as long-term rapamycin is now known to inhibit of mTOR Complex 2 which has other effects including development of glucose intolerance (Hughes and Kennedy 2012). Perhaps inhibition of mTOR complex 2 explains why our aged mice on LT-eRapa were slightly more susceptible to infection than those on ST-eRapa? Of note, the effects of rapamycin are complex and most likely impact infectious disease susceptibility/resistance in a variety of ways simultaneously; some of which are indirect. For example Chen et al. showed that that 8 weeks of rapamycin administration increased bone marrow production of B-cells in aged mice and enhanced their ability to respond to influenza vaccination. This improved aged mouse survival following influenza challenge (Chen et al., 2009).
In summary, we have determined that prolonged eRapa has no detrimental effect for aged mice against S. pneumoniae. This was a somewhat a surprising result given that rapamycin is generally considered to be immunosuppressive and aged mice are exquisitely susceptible to pneumococcal pneumonia. Our findings suggest that the acute antibacterial immune response to this pathogen is not seriously compromised and the beneficial effects of therapeutic rapamycin, seem to come from reduced cellular senescence in vivo and reduced lung pathology. Although our studies used a small number of mice and extrapolating mice data to humans is risky, they do support the possibility that rapamycin can be used as a human therapeutic. Importantly, the underlying mechanisms responsible for the differential effect of eRapa with other pathogens need to be evaluated empirically.
HIGHLIGHTS.
Enteric rapamycin (eRapa) protected aged mice against pneumonia-related death
Aged mice on eRapa had reduced tissue damage versus controls during pneumonia
eRapa had no effect on bacterial burden or the tested cytokine & chemokine response
eRapa reduced lung levels of proteins co-opted by pathogenic bacteria for infection
eRapa reduced levels of pRB and p21 in the lungs of aged mice
Acknowledgments
Support for this project was from NIH AI083387 to SB, AG0123823 to RM, and AG033274 and AG036613 - Project 5 to CJO.
Footnotes
AUTHOR CONTRIBUTIONS
CAH and VEM together completed the presented work. SVR and RM at University of Michigan provided material support in regards to lung samples from mice administered low-, mid- and high- eRapa. SNA, SB and CJO were involved in experimental design and oversaw the completion of the research project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert S, Serova M, Dreyer C, Sablin MP, Faivre S, Raymond E. New inhibitors of the mammalian target of rapamycin signaling pathway for cancer. Expert Opin Investig Drugs. 2010;19:919–930. doi: 10.1517/13543784.2010.499121. [DOI] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avadhanula V, Rodriguez CA, Ulett GC, Bakaletz LO, Adderson EE. Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect Immun. 2006;74:830–838. doi: 10.1128/IAI.74.2.830-838.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier M, Oliver A, Rao J, Hanna SL, Goldberg JB, Alberti S. Novel phosphorylcholine-containing protein of Pseudomonas aeruginosa chronic infection isolates interacts with airway epithelial cells. J Infect Dis. 2008;197:465–473. doi: 10.1086/525048. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Majumder S, Deng JJ, Bai Y, Thornton FB, Oddo S. Rapamycin rescues TDP-43 mislocalization and the associated low molecular mass neurofilament instability. J Biol Chem. 2009;284:27416–27424. doi: 10.1074/jbc.M109.031278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child ES, Mann DJ. The intricacies of p21 phosphorylation: protein/protein interactions, subcellular localization and stability. Cell Cycle. 2006;5:1313–1319. doi: 10.4161/cc.5.12.2863. [DOI] [PubMed] [Google Scholar]
- Conners R, Hill DJ, Borodina E, Agnew C, Daniell SJ, Burton NM, Sessions RB, Clarke AR, Catto LE, Lammie D, Wess T, Brady RL, Virji M. The Moraxella adhesin UspA1 binds to its human CEACAM1 receptor by a deformable trimeric coiled-coil. EMBO J. 2008;27:1779–1789. doi: 10.1038/emboj.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle. 2008;7:3355–3361. doi: 10.4161/cc.7.21.6919. [DOI] [PubMed] [Google Scholar]
- Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8:1888–1895. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- Fielhaber JA, Carroll SF, Dydensborg AB, Shourian M, Triantafillopoulos A, Harel S, Hussain SN, Bouchard M, Qureshi ST, Kristof AS. Inhibition of Mammalian Target of Rapamycin Augments Lipopolysaccharide-Induced Lung Injury and Apoptosis. J Immunol. 2012 doi: 10.4049/jimmunol.1003655. [DOI] [PubMed] [Google Scholar]
- Finckenberg P, Mervaala E. Novel regulators and drug targets of cardiac hypertrophy. J Hypertens. 2010;28(Suppl 1):S33–38. doi: 10.1097/01.hjh.0000388492.73954.0b. [DOI] [PubMed] [Google Scholar]
- Groth CG, Backman L, Morales JM, Calne R, Kreis H, Lang P, Touraine JL, Claesson K, Campistol JM, Durand D, Wramner L, Brattstrom C, Charpentier B. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation. 1999;67:1036–1042. doi: 10.1097/00007890-199904150-00017. [DOI] [PubMed] [Google Scholar]
- Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa E, Boyd AR, Orihuela CJ. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J Infect Dis. 2009;200:546–554. doi: 10.1086/600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KJ, Kennedy BK. Cell biology. Rapamycin paradox resolved. Science. 2012;335:1578–1579. doi: 10.1126/science.1221365. [DOI] [PubMed] [Google Scholar]
- Husain S, Alexander BD, Munoz P, Avery RK, Houston S, Pruett T, Jacobs R, Dominguez EA, Tollemar JG, Baumgarten K, Yu CM, Wagener MM, Linden P, Kusne S, Singh N. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin Infect Dis. 2003;37:221–229. doi: 10.1086/375822. [DOI] [PubMed] [Google Scholar]
- Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202–209. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiling JA, Tamamori-Adachi M, Sexton AN, Jeyapalan JC, Munoz-Najar U, Peterson AL, Manivannan J, Rogers ES, Pchelintsev NA, Adams PD, Sedivy JM. Age-associated increase in heterochromatic marks in murine and primate tissues. Aging Cell. 2011;10:292–304. doi: 10.1111/j.1474-9726.2010.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany NY) 2010;2:924–935. doi: 10.18632/aging.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley MM, Harrison LH, Schaffner W, Reingold A, Bennett NM, Hadler J, Cieslak PR, Whitney CG. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–2051. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- Manning AL, Dyson NJ. pRB, a tumor suppressor with a stabilizing presence. Trends Cell Biol. 2011;21:433–441. doi: 10.1016/j.tcb.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minino AM, Heron MP, Smith BL. Deaths: preliminary data for 2004. Natl Vital Stat Rep. 2006;54:1–49. [PubMed] [Google Scholar]
- O’Brien LM, Walsh EJ, Massey RC, Peacock SJ, Foster TJ. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonization. Cell Microbiol. 2002;4:759–770. doi: 10.1046/j.1462-5822.2002.00231.x. [DOI] [PubMed] [Google Scholar]
- Orihuela CJ, Gao G, Francis KP, Yu J, Tuomanen EI. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J Infect Dis. 2004;190:1661–1669. doi: 10.1086/424596. [DOI] [PubMed] [Google Scholar]
- Orihuela CJ, Mahdavi J, Thornton J, Mann B, Wooldridge KG, Abouseada N, Oldfield NJ, Self T, Ala’Aldeen DA, Tuomanen EI. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest. 2009;119:1638–1646. doi: 10.1172/JCI36759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuela CJ, Tuomanen E. Streptococcus pneumoniae: Invasion and Inflammation. In: Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI, editors. Gram-Positive Pathogens. Washington D.C: ASM Press; 2006. [Google Scholar]
- Shivshankar P, Boyd AR, Le Saux CJ, Yeh IT, Orihuela CJ. Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell. 2011;10:798–806. doi: 10.1111/j.1474-9726.2011.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivshankar P, Sanchez C, Rose LF, Orihuela CJ. The Streptococcus pneumoniae adhesin PsrP binds to Keratin 10 on lung cells. Mol Microbiol. 2009;73:663–679. doi: 10.1111/j.1365-2958.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS ONE. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li C, Shu Y, Ju X, Zou Z, Wang H, Rao S, Guo F, Liu H, Nan W, Zhao Y, Yan Y, Tang J, Zhao C, Yang P, Liu K, Wang S, Lu H, Li X, Tan L, Gao R, Song J, Gao X, Tian X, Qin Y, Xu KF, Li D, Jin N, Jiang C. Inhibition of autophagy ameliorates acute lung injury caused by avian influenza A H5N1 infection. Sci Signal. 2012;5:ra16. doi: 10.1126/scisignal.2001931. [DOI] [PubMed] [Google Scholar]
- Swords WE, Buscher BA, Ver Steeg K, II, Preston A, Nichols WA, Weiser JN, Gibson BW, Apicella MA. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol Microbiol. 2000;37:13–27. doi: 10.1046/j.1365-2958.2000.01952.x. [DOI] [PubMed] [Google Scholar]
- Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, Heidelberg J, DeBoy RT, Haft DH, Dodson RJ, Durkin AS, Gwinn M, Kolonay JF, Nelson WC, Peterson JD, Umayam LA, White O, Salzberg SL, Lewis MR, Radune D, Holtzapple E, Khouri H, Wolf AM, Utterback TR, Hansen CL, McDonald LA, Feldblyum TV, Angiuoli S, Dickinson T, Hickey EK, Holt IE, Loftus BJ, Yang F, Smith HO, Venter JC, Dougherty BA, Morrison DA, Hollingshead SK, Fraser CM. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- Tirado OM, Mateo-Lozano S, Notario V. Rapamycin induces apoptosis of JN-DSRCT-1 cells by increasing the Bax : Bcl-xL ratio through concurrent mechanisms dependent and independent of its mTOR inhibitory activity. Oncogene. 2005;24:3348–3357. doi: 10.1038/sj.onc.1208471. [DOI] [PubMed] [Google Scholar]
- Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation. 2006;81:1234–1248. doi: 10.1097/01.tp.0000219703.39149.85. [DOI] [PubMed] [Google Scholar]
- Yan Y, Silvennoinen-Kassinen S, Leinonen M, Saikku P. Rapamycin can inhibit the development of Chlamydia pneumoniae, which might partly contribute to the prevention of in-stent restenosis. Cardiovasc Drugs Ther. 2010;24:189–195. doi: 10.1007/s10557-010-6238-8. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]