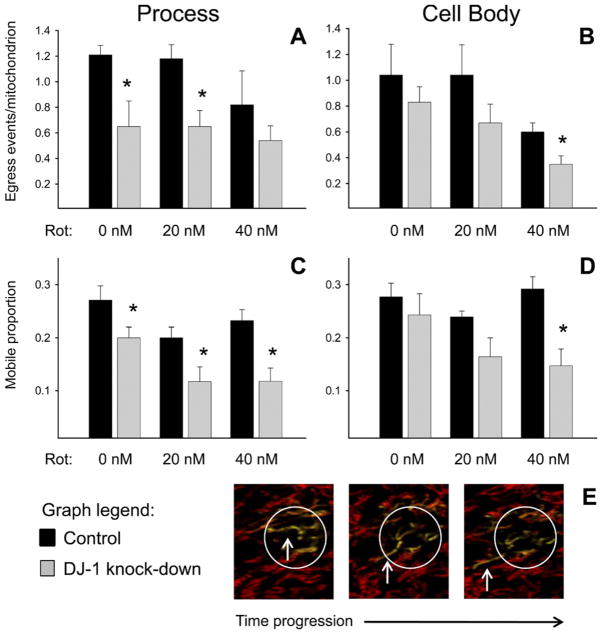
Fig. 2.
DJ-1 knock-down reduced mitochondrial motility predominantly at cellular processes in astrocyte-enriched cultures. Control (black bars) and DJ-1 knock-down (gray bars) astrocytes were co-transfected with mtDsRed2 and mtPAGFP, and then treated for 24 h with 0, 20, or 40 nM rotenone. Mitochondrial egress events (mitochondrial movements across an ROI boundary, (A–B)) and mobile mitochondria (within an ROI, (C–D)) were counted and then normalized to the total number of mitochondria within the same ROI. Asterisks (*) represent P<0.05 for same-treatment comparisons between control and DJ-1 knock-down astrocytes by paired t-tests. Mean±SE shown, n=6. (A) In astrocyte processes, DJ-1 knock-down reduced mitochondrial egress in untreated and 20 nM rotenone treated cells. (B) In astrocyte cell bodies, DJ-1 knock-down caused a trend towards reduced mitochondrial egress that was not significant until the cells were treated with 40 nM rotenone. (C) In astrocyte processes, DJ-1 knock-down reduced the mitochondrial mobile proportion under all conditions of treatment. (D) In astrocyte cell bodies, DJ-1 knock-down caused a trend towards reduced mitochondrial mobile proportion that was not significant until the cells were treated with 40 nM rotenone. (E) Sequential photomicrograph frames of a mobile photoactivated mtPAGFP+ (green fluorescent) mitochondrion that leaves a simulated laser-photoactivated ROI. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.