Abstract
Objective
To compare cerebral blood flow (CBF) autoregulation in patients undergoing continuous flow left ventricular assist device (LVAD) implantation with that in patients undergoing coronary artery bypass graft (CABG) surgery.
Design
Prospective, observational, controlled study.
Setting
Academic medical center.
Participants
Fifteen patients undergoing LVAD insertion and 10 patients undergoing CABG surgery.
Measurements and Main Results
Cerebral autoregulation was monitored with transcranial Doppler and near-infrared spectroscopy (NIRS). A continuous, Pearson's correlation coefficient was calculated between mean arterial pressure (MAP) and CBF velocity, and between MAP and NIRS data rendering the variables mean velocity index (Mx) and cerebral oximetry index (COx), respectively. Mx and COx approach zero when autoregulation is intact (no correlation between CBF and MAP), but approach 1 when autoregulation is impaired. Mx was lower during and immediately after cardiopulmonary bypass (CPB) in the LVAD group than it was in the CABG surgery patients, indicating better preserved autoregulation. Based on COx monitoring, autoregulation tended to be better preserved in the LVAD group than in the CABG group immediately after surgery (p=0.0906). On postoperative day 1, COx was lower in LVAD patients than in CABG surgery patients, again indicating preserved CBF autoregulation (p=0.0410). Based on COx monitoring, 3 (30%) of the CABG patients had abnormal autoregulation (COx ≥ 0.3) on the first postoperative day but none of the LVAD patients had this abnormality (p=0.037).
Conclusion
These data suggest that CBF autoregulation is preserved during and immediately after surgery in patients undergoing LVAD insertion.
INTRODUCTION
Continuous flow left ventricular assist devices (LVADs) are increasingly used in patients with end-stage left ventricular failure both as destination therapy and as a bridge to transplant.1,2 Despite their benefits for improving functional capacity, quality of life, and survival, LVAD use is associated with a risk for stroke in 5% to 12% of patients.3–6 Between 33% and 63% of strokes occur within 48 hours of device implantation.4,6 Understanding the mechanism of stroke in patients with LVADs has important public health implications in light of the rising prevalence of heart failure in the general population for whom LVAD therapy may be considered.
Cerebral blood flow (CBF) autoregulation normally ensures a steady supply of oxygenated blood to the brain over a range of blood pressures. Impairment of autoregulation results in CBF that is pressure-passive or directly dependent on blood pressure. The result might be cerebral hypoperfusion with low blood pressure or cerebral hyperemia with high blood pressure; both conditions predispose a patient to brain injury.7 Impairment of CBF autoregulation is increasingly recognized to be associated with poor outcomes in patients with neurologic diseases, including traumatic brain injury.8 We have previously observed a relationship between impaired CBF autoregulation during nonpulsatile cardiopulmonary bypass (CPB) and perioperative stroke.2,9,10 Recent data obtained in awake patients with pulsatile LVADs have suggested that some aspects of CBF autoregulation may be impaired after device implantation.11 That study was performed 7 days after surgery; thus, it did not provide data regarding autoregulation in the immediate perioperative period, when the risk of neurologic injury is high. Restoration of normal circulatory flow with an LVAD in patients with preexisting low cardiac output has been further suggested to overwhelm autoregulatory mechanisms that contribute to postoperative neurologic complications, although conclusive evidence confirming this hypothesis has not been provided.3
Cerebral autoregulation can be measured continuously in real time by monitoring the correlation between low-frequency changes in CBF in response to spontaneous fluctuations in blood pressure.8 Transcranial Doppler (TCD) is usually used to monitor CBF velocity for this purpose in anesthetized or sedated patients, but its use is limited in awake patients by movement artifact and other known limitations.8 We have previously reported data from laboratory and clinical investigations showing that near-infrared spectroscopy (NIRS) can be used for monitoring autoregulation, because low frequency changes in cerebral oximetry signals are coherent with similar frequency changes in CBF velocity.12–14 Using NIRS for autoregulation monitoring has many advantages that overcome the limitations of TCD; importantly, it allows for continuous monitoring despite patient movement.
The purpose of this pilot study was to assess CBF autoregulation using TCD and NIRS in patients undergoing continuous flow LVAD implantation and to compare maintenance of autoregulation in such patients with that in a cohort of patients undergoing cardiac surgery with CPB.
METHODS
All procedures were approved by The Johns Hopkins Medical Institutions Investigational Review Board. Each patient provided written informed consent for the study. The patients were those with New York Heart Association functional class IV heart failure who were either under consideration for heart transplantation or who were not otherwise candidates for transplantation.6 A group of 10 patients undergoing elective coronary artery bypass graft (CABG) surgery during the same time period of this study served as controls. These patients were specifically enrolled for this study and were not part of our prior reports. Patients were excluded for 1) preexisting renal dialysis; 2) emergency surgery; 3) inadequate temporal window for TCD monitoring; or 4) insufficient slow wave activity for autoregulation monitoring. The CABG patients underwent surgery during the same time period as the LVAD patients and were selected based on availability of personnel and equipment.
Intraoperative Care
The patients were administered midazolam, fentanyl, and isoflurane for anesthesia and pancuronium for skeletal muscle relaxation by methods previously described.2,9,10 Standard monitoring for patients undergoing cardiac surgery was used, including indwelling radial artery blood pressure monitoring. Isoflurane was administered via the membrane oxygenator during CPB but usually in concentrations of <1%. After CPB, isoflurane concentrations were maintained at <0.5% and then discontinued when a propofol infusion was started for postoperative sedation. Mechanical ventilation was adjusted to maintain normocarbia based on end-tidal CO2 measurement and arterial blood gas measurement. Nonpulsatile, continuous-flow LVADs (HeartMate II LVAS, Thoratec, Corporation, Pleasanton, CA) were implanted by methods previously described.6 Implantation of the device was via a median sternotomy and with the use of CPB. The latter was with a nonocclusive roller pump, a membrane oxygenator, and a 27-μm arterial line filter with flow between 2.0 and 2.4 L/min/m2. Gas flow to the oxygenator was controlled to maintain normocarbia based on arterial PaCO2 results or continuous in-line arterial blood gas monitoring. Clinical management of CPB was based on institutional standards, including blood pressure targets and rewarming rate. The patients were managed with α-stat pH management. An epinephrine infusion was started before separation from CPB in all patients and continued until it was weaned off based on cardiovascular performance.
Postoperative Care
The patients received routine institutional postperative care that included initiation of anticoagulation therapy 48 hours after surgery with either warfarin or heparin.6 Postoperative variables, including complications that affect organ systems, were collected during the hospitalization. Definitions of complications were based on the Society of Thoracic Surgery nomenclature.15 Encephalopathy was defined as confusion, agitation, or change in mental status as identified by nurses using observational methods previously reported.16 Acute kidney injury was diagnosed based on the RIFLE criteria.17
Autoregulation Monitoring
The patient's right and left middle cerebral arteries were monitored with TCD (Doppler Box, DWL, Compumedics, USA, Charlotte, NC) via two 2.5-MHz transducers fitted on a headband. NIRS monitoring was carried out via two self-adhesive probes that were affixed to the right and left forehead and attached to an Invos™ cerebral oximeter (Covidien, Inc, Boulder, CO). Arterial pressure from an indwelling radial artery cannula and TCD and NIRS signals were digitized at 58 Hz with an analog-to-digital converter (DT9804, Data Translation, Marlboro, MA) using ICM+ software (Cambridge University, UK) according to methods previously described.2,9,12,13 A continuous, moving Pearson correlation coefficient was calculated between mean arterial pressure (MAP) and CBF velocity and between MAP and NIRS to generate the variables mean velocity index (Mx) and cerebral oximetry index (COx), respectively. Paired, 10-second averaged values over 300-second epochs were used for each calculation, incorporating 30 data points. CBF autoregulation was monitored continuously during surgery and then for a minimum of 55 minutes on the first postoperative day after tracheal extubation. Postoperative autoregulation was monitored with COx only because of patient movement that precludes continuous TCD signal acquisition.
Slow Wave Characterization
Agreement of autoregulation metrics by different modalities is a function of coherence between the primary signals in the slow wave bandwidth.18 It is reasonable to question whether patients on continuous-flow LVAD support have spontaneous hemodynamic slow wave activity sufficient to render an autoregulation metric. We therefore characterized slow wave activity from an individual COx value using the recordings from the intensive care unit. Slow wave amplitude was approximated as the maximum – minimum MAP (ΔMAP) value in 300-second epochs, updated at the same 60-second interval used for COx measurements. The ΔMAP from every 300-second epoch was recorded for each subject, and the median ΔMAP was identified for comparison between LVAD and CABG patients. The distribution of the median ΔMAP in the two groups was used to define excessive slow wave amplitude of 30 mmHg (>2 standard deviations for both groups). The percentage of epochs with excessive slow wave amplitude (>30 mmHg) was recorded. The percentage of epochs with insufficient slow wave power (<5 mmHg blood pressure change in the slow wave bandwidth) was recorded for each subject.
Statistical Analysis
Because this was a pilot study, our intent was to obtain preliminary data to allow sample size determination for subsequent study. We elected to enroll 10 CABG patients based on our experience that this number would provide adequate data for this purpose. The number of LVAD patients enrolled reflected consecutive patients undergoing this procedure during this time period. Dichotomous data were evaluated with Fishers Exact Test. Continuous data were evaluated with analysis of variance, and the Bonferroni multiple comparison test was used when needed. Right and left TCD and NIRS recordings were combined for analysis. Time-averaged values for Mx and COx obtained at each perioperative measurement period were compared with baseline measurements. Patients were categorized based on the presence or absence of impaired autoregulation during each period. The exact Mx or COx associated with impaired autoregulation is not clear. For this study we defined impaired CBF autoregulation as a time-average Mx ≥ 0.4 or COx ≥ 0.3 based on prior animal and clinical investigations.8,9,13,19 Slow wave characterizations were compared between the LVAD and CABG groups with a Mann Whitney U test. Analysis was performed with GraphPad Prism software (GraphPad Software, Inc, La Jolla, CA) or Stata software (version 9.0, Stata Corp, College Station, TX).
RESULTS
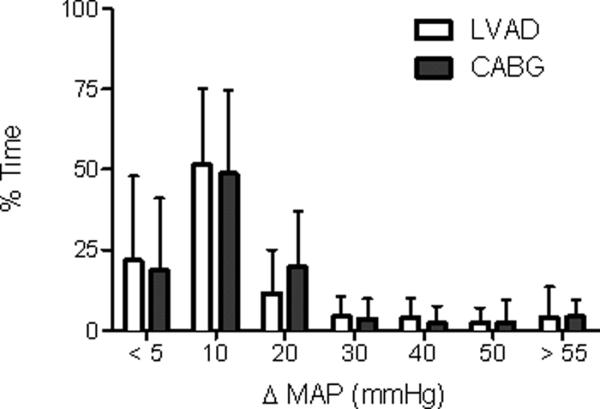
Of the 17 patients undergoing LVAD insertion, 2 did not exhibit slow waves of NIRS data on postoperative day 1. These patients were not included in the analysis. The distribution of slow wave activity was similar between the LVAD and CABG groups on postoperative day 1 (Fig 1). The median ΔMAP was 11 mmHg ± 8 mmHg for the LVAD group and 11 mmHg ± 7 mmHg for the CABG group (p = 0.80). The percentage of epochs with inadequate (<5 mmHg) or excessive (>30 mmHg) ΔMAP in the slow wave bandwidth was 20% ± 23% and 18% ± 21% (p = 0.68) and 9% ± 14% and 8% ± 13% (p = 0.50) in the LVAD and CABG groups, respectively. The patient characteristics and surgical data for the patients are listed in Table 1. There were few differences between surgical groups. Three LVAD patients had a history of coronary artery disease, the remainder had idiopathic cardiomyopathy. More patients in the LVAD group were receiving an epinephrine infusion during the postoperative day 1 monitoring session than in the CABG group. There were no differences in patient outcomes between groups after surgery (Table 2).The temperature nadir during CPB was lower in the CABG patients than in the LVAD patients (32.5±2.2°C versus 35.2±1.7°C, p = 0.0014). Laboratory results during and after CPB are listed in Table 3. Compared with the LVAD group, pH was lower during and after CPB in the CABG group. After CPB, PaO2 was lower in the CABG group compared the LVAD group. There are no blood gas or hemoglobin data during the postoperative monitoring session.
Fig 1.
Slow wave activity is comparable in patients undergoing left ventricular assist device (LVAD) insertion and those undergoing coronary artery bypass graft (CABG) surgery. Slow wave power was approximated by change in mean arterial pressure (ΔMAP) within the slow wave bandwidth. The distribution of epochs (% time) across ΔMAP is shown for LVAD and CABG subjects separately (Group mean and S.D.). Presence of LVAD support did not associate with any statistically significant change in ΔMAP.
Table 1.
Medical and surgical information for patients undergoing left ventricular assist device (LVAD) insertion or coronary artery bypass graft (CABG) surgery
| Variable | LVAD Patients (n=15) | CABG Patients (n=10) | p Value LVAD vs. CABG |
|---|---|---|---|
| Age (years) (mean±SD) | 60±10 | 62±10 | 0.8784 |
| Gender | 0.085 | ||
| Male | 10 (67%) | 10 (100%) | |
| Female | 5 (33%) | 0 | |
| Hypertension | 7 (47%) | 8 (80%) | 0.211 |
| Diabetes | 6 (40%) | 6 (60%) | 0.428 |
| Chronic obstructive pulmonary disease | 4 (27%) | 1 (10%) | 0.615 |
| Peripheral vascular disease | 0 | 2 (20%) | 0.150 |
| Current tobacco smoker | 1 (7%) | 1 (10%) | 1.0 |
| Congestive heart failure | 15 (100%) | 2 (20%) | <0.0001 |
| Prior myocardial infarction | 0 | 1 (10%) | 0.400 |
| Prior stroke | 1 (7%) | 0 | 1.0 |
| Left carotid stenosis | 0.559 | ||
| 50–70% | 11 (73%) | 5 (50%) | |
| 70–90% | 1 (7%) | 1 (10%) | |
| >90% | 0 | 0 | |
| Right carotid stenosis | 0.378 | ||
| 50–70% | 12 (80%) | 6 (60%) | |
| 70–90% | 0 | 0 | |
| >90% | 0 | 0 | |
| Left ventricular ejection fraction <30% | 15 (100%) | 2 (20%) | <0.0001 |
| Medications | |||
| Aspirin | 7 (47%) | 9 (90%) | 0.040 |
| Beta-blockers | 7 (47%) | 3 (30%) | 0.678 |
| “Statin” drugs | 2 (13%) | 6 (60%) | 0.028 |
| Ca2+ channel blockers | 1 (7%) | 2 (20%) | 0.543 |
| ACE inhibitors | 4 (27%) | 4 (40%) | 0.667 |
| Dobutamine | 2 (13%) | 0 | 0.500 |
| Milrinone | 9 (60%) | 0 | 0.003 |
| Prior cardiac surgery | 4 (27%) | 1 (10%) | 0.615 |
| Cardiopulmonary bypass duration (min) | 79±33 | 89±24 | 0.4370 |
| Aortic cross-clamp duration (min) | - | 56±16 | |
| Epinephrine Infusion During | 10 | 1 | 0.014 |
| Postoperative Day 1 Monitoring |
Abbreviation: ACE, angiotensin-converting enzyme.
Table 2.
Postoperative in-hospital outcomes for patients undergoing left ventricular assist device LVAD) insertion or coronary artery bypass graft (CABG) surgery
| Variable | LVAD Patients (n=15) | CABG Patients (n=10) | p Value |
|---|---|---|---|
| Stroke | 1 (7%) | 0 | 1.0 |
| Encephalopathy | 3 (20%) | 0 | 0.250 |
| Mechanical ventilation > 72 h | 2 (13%) | 0 | 0.500 |
| Acute kidney injury within 48 h of surgery | 1 (7%) | 3 (30%) | 0.267 |
| Mortality | 1 (7%) | 0 | 1.0 |
| Postoperative atrial fibrillation | 1 (7%) | 1 (10%) | 0.267 |
Table 3.
Laboratory results obtained during surgery for LVAD and CABG surgery patients. Data are presented as mean±SD.
| LVAD Group (n=15) | CABG Group (n-10) | P-Value | |
|---|---|---|---|
| pH during CPB | 7.40±0.03 | 7.37±0.02 | 0.0284 |
| pH after CPB | 7.41±0.04 | 7.34±0.03 | 0.0343 |
| PaCO2 during CPB (mmHg) | 42±3 | 42±2 | 0.8066 |
| PaCO2 after CPB (mmHg) | 37±4 | 39±5 | 0.3699 |
| PaO2 during CPB (mmHg) | 254±38 | 252±14 | 0.7706 |
| PaO2 after CPB (mmHg) | 318±92 | 192±91 | 0.0020 |
| Hemoglobin during CPB (gm/dL) | 9.0±1.1 | 9.2±1.1 | 0.7454 |
| Hemoglobin after CPB (gm/dL) | 8.8±1.0 | 8.9±1.2 | 0.8193 |
Blood pressure, cerebral blood flow velocity, and cerebral oxygen saturation (rSO2) results are listed in Table 4. Blood pressure was lower after CPB and during monitoring on the first postoperative day. Average CBF velocity tended to be higher in the CABG patients than in the LVAD patients, although the difference was significant only during CPB. In both groups, CBF velocity was higher after CPB than before CPB. rSO2 values were lower in the LVAD group than in the CABG surgery group before CPB, but they were higher on the first postoperative day.
Table 4.
Cerebral blood flow (CBF) velocity and cerebral oxygen saturation (rSO2) for patients undergoing left ventricular assist device (LVAD) insertion or coronary artery bypass graft (CABG) surgery. Data are listed as mean±SD.
| LVAD Patients (n=15) | CABG Patients (n=10) | p Value | |
|---|---|---|---|
| Average Mean Arterial Blood Pressure (mmHg) |
|||
|
| |||
| Before CPB | 78±10 | 83±9 | 0.2071 |
|
| |||
| During CPB | 66±9 | 70±10 | 0.1971 |
|
| |||
| After CPB | 86±8 | 72±9 | 0.0001 |
|
| |||
| Postoperative day 1 | 84±16 | 69±13 | 0.0351 |
|
| |||
| Average CBF velocity (cm/sec) | |||
| Before CPB | 30.1±6.3 | 34.4±10.6 | 0.2456 |
| During CPB | 31.8±7.4 | 41.1± 5.9 | 0.0045 |
| After CPB | 40.8±12.6* | 47.8±7.3** | 0.1364 |
| Average rSO2 (%) | |||
| Before CPB | 54.4±10.7 | 63.2±11.6 | 0.0686 |
| During CPB | 53.6±9.7 | 51.7±10.9 | 0.6528 |
| After CPB | 57.82±7.4 | 51.8±10.1 | 0.1072 |
| Postoperative day 1 | 60.3±8.8 | 51.0±11.3 | 0.0351 |
Abbreviation: CPB, cardiopulmonary bypass.
p = 0.054 vs before CPB;
p = 0.007 vs before CPB.
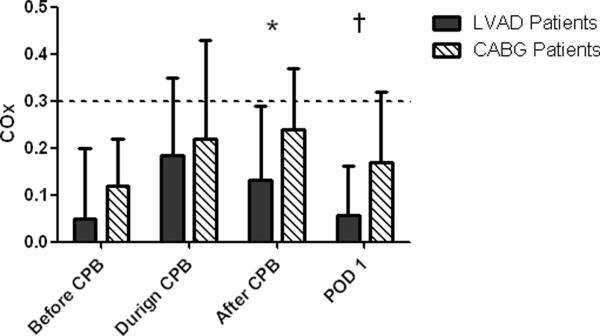
Cerebral autoregulation results are shown in Table 5 and Figure 2. For each group, Mx was higher during CPB than before CPB, a change that is compatible with some impaired autoregulation. The Mx was higher in the CABG surgery group than in the LVAD group both during and after CPB, indicating more perturbed autoregulation. After CPB, Mx in the LVAD group decreased to a level that was lower than that during CPB, but no different than the pre-CPB value. In the CABG surgery group, Mx was higher after CPB than before CPB but was not different than the measurement during CPB. COx monitoring indicated no difference in autoregulation between groups during CPB, but autoregulation tended to be more preserved in the LVAD group than in the CABG group after CPB (p = 0.0906). On postoperative day 1, COx was lower in the LVAD group than in the CABG surgery group, indicating preserved CBF autoregulation (p = 0.0410).
Table 5.
Transcranial Doppler autoregulation monitoring results during surgery
| LVAD Patients (n=15) | CABG Patients (n=10) | p value | |
|---|---|---|---|
| Mx before CPB | 0.04±0.09 | 0.10±0.13 | 0.1781 |
| Mx during CPB | 0.30±0.13* | 0.42±0.14* | 0.0551 |
| Mx after CPB | 0.08±0.14†# | 0.31±0.13‡§ | 0.0009 |
NOTE: Mean velocity index (Mx) refers to the correlation coefficient between mean arterial pressure and cerebral blood flow velocity. When cerebral blood flow is autoregulated, Mx approaches zero; impaired autoregulation is indicated by an Mx that approaches 1. Abnormal autoregulation in this study was defined as an Mx ≥ 0.4 at all blood pressures.
Abbreviations: LVAD, left ventricular assist device; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass.
p<0.0001 vs before CPB;
p=0.001 vs during CPB;
p=0.878 vs before CPB;
p= 0.295 vs during CPB;
p=0.008 vs before CPB.
Fig 2.
Cerebral oximetry index (COx) results for patients undergoing left ventricular assist device (LVAD) insertion or coronary artery bypass graft (CABG) surgery. COx values were collected before, during, and after cardiopulmonary bypass (CPB) and on the first postoperative day (POD1). COx is the linear correlation coefficient between cerebral oximetry readings as a surrogate for cerebral blood flow and mean arterial pressure. When autoregulated, COx approaches zero, but when autoregulation is impaired COx approaches one, indicating a direct correlation between COx and MAP. The dashed line at a COx value of 0.3 indicates the threshold for defining impaired autoregulation or the state where cerebral blood flow is pressure passive. *p = 0.0906; †p = 0.0410.
Based on Mx monitoring, 7 of 10 (70%) CABG surgery patients had abnormal autoregulation (Mx ≥ 0.4) during CPB compared with 5 of 15 (33%) LVAD patients (p = 0.111). After CPB, 4 of 10 (40%) CABG patients had abnormal autoregulation whereas abnormal autoregulation was not observed in any of the LVAD patients (p = 0.020). Based on COx monitoring, 3 of 10 (30%) CABG patients had abnormal autoregulation (COx ≥ 0.3) on the first postoperative day but none of the LVAD patients had this abnormality (p = 0.037).
DISCUSSION
In this study we found that CBF autoregulation is preserved in patients undergoing continuous flow LVAD insertion during surgery and on the first postoperative day, suggesting that the ability of the brain to constrain CBF over a range of blood pressures is preserved. The relative merits of pulsatile versus nonpulsatile extracorporeal flow have been debated for decades with reference to CPB for cardiac surgery. Multiple studies in patients undergoing nonpulsatile CPB have demonstrated preserved CBF autoregulation when alpha-stat pH management is used.20 Further, studies in animals failed to find an effect of nonpulsatile CPB on global CBF or regional oxygen saturation.21 Nonetheless, in humans pulsatile CPB flow is associated with decreased neurohumoral activation, lower vascular resistance, higher visceral blood flow, and improved renal and liver function compared with nonpulsatile flow.22 The higher energy imparted by pulsatile flow provides more efficient distribution of blood flow to the microcirculation than does nonpulsatile flow and leads to less edema, improved brain oxygenation after circulatory arrest, improved blood flow to ischemic brain regions, lower neuropathologic abnormalities in ischemic penumbral regions, less inflammation, and other benefits.22 Findings are conflicting with regard to clinical benefit of pulsatility on neurologic outcomes, but the data are limited.22–26
Impaired autoregulation has been implicated in promoting cerebral hyperemia with restoration of circulatory flow after LVAD insertion. Leitz et al3 studied 69 patients who had undergone insertion of a pulsatile LVAD and found neurologic dysfunction in 19 (27.5%) patients, including encephalopathy (n=11) and coma (n=3). They showed that an increase in cardiac index from the preoperative value (relative risk, 1.33 per 25% cardiac index increase; p = 0.01) and prior CABG surgery (relative risk, 4.53; p = 0.02) were predictors of neurologic complications. Further, reducing LVAD flow led to improvement in neurologic symptoms in 16 of 19 patients. Despite having preserved autoregulation, patients in the LVAD group in our study had a higher rate of neurologic complications than did those in the CABG group. However, the small number of patients in our study does not allow for us to confirm or refute any role of cerebral hyperemia in postoperative neurologic complications after LVAD insertion.
Our findings that nonpulsatile LVAD flow had no effect on autoregulation are in contrast to those of Bellapart et al,11 who evaluated 5 patients for an average of 7 days after pulsatile LVAD implantation. A group of 5 patients matched for age and comorbidities who had low cardiac output that required inotropic drugs served as controls. In that study, transfer function analysis from 5-minute recordings was performed on low-frequency components (0.02 to 0.35 Hz) of MAP and TCD-measured CBF velocity.27 These investigators found no difference in gain or phase results between LVAD patients and controls, but low-frequency coherence was higher in LVAD patients than in controls (mean ± SD, 0.65 ± 0.16 vs. 0.38 ± 0.19, p = 0.04). The authors noted that 2 LVAD patients with the highest coherence (~0.8), indicating correlation between MAP and CBF, had higher spectral power in MAP than other patients. Increased MAP may result in higher CBF velocity, which contributes to higher coherence due to better signal-to-noise ratio and not necessarily because of reduced autoregulation.8,28
In our study, we monitored autoregulation with NIRS using previously validated methods through which we and others have found high coherence between cerebral oximetry and CBF velocity at frequencies <0.04 Hz, suggesting that the former is a clinically reliable surrogate for CBF.12,13 Our method of autoregulation monitoring is based on time-domain rather than frequency-domain methods such as those used in the study by Bellapart et al.11 An advantage of our approach is that, unlike frequency-domain monitoring, time-domain autoregulation monitoring does not require assumptions of stationarity, a condition usually not present during surgery or in the intensive care units.8 Further, our methods do not require multiple abrupt episodes of hypotension for measuring CBF responses that would not be well tolerated in patients after LVAD insertion. A limitation of time-domain autoregulation monitoring is that the signal-to-noise ratio is less than with autoregulation testing in which blood pressure is manipulated. Focusing on low-frequency (0.003 to 0.04 Hz) fluctuations in CBF velocity representative of autoregulatory compensations and recording samples >30 minutes improve the signal-to-noise ratio.8 Our methods assess autoregulatory compensations to mean blood pressure and therefore are not affected by a nonpulsatile state. We have previously demonstrated high coherence between slow waves of MAP and cerebral oximetry in animals and in patients during CPB.12,13 In this study we confirmed the presence of slow waves as a necessary prerequisite for autoregulation monitoring after surgery, supporting our methods in LVAD patients.
Our findings are associated with several limitations. More women were in the LVAD group than in the CABG patients. Further, there was a trend toward a higher prevalence of hypertension and peripheral vascular disease in the CABG group than in the LVAD group. The effect of patient sex on autoregulation is not clearly defined, but the higher rate of widespread atherosclerosis in the CABG patients may indicate that patients in this group had a higher rate of cerebral vascular disease than patients in the LVAD group. Additionally, during CPB, CBF velocity was higher in the CABG surgery patients than in the LVAD patients. High TCD-detected velocity may indicate intracranial arterial stenosis in some of these patients.29,30 Thus, the better preserved autoregulation observed in the LVAD group may indicate a higher prevalence of cerebral vascular disease in the CABG surgery patients. Temperature during CPB was lower in the CABG surgery patients than in the LVAD patients. Using methods similar to those used here, we previously found that autoregulation was preserved during mild hypothermia, although the threshold was shifted to the left.31 Perhaps more important, we have previously found a high rate of autoregulation impairment during patient rewarming on CPB.9 Thus, the lower body temperature in the CABG surgery patients might have necessitated greater rewarming that in turn contributed to impaired autoregulation at least during the CPB period. It seems unlikely that autoregulatory dysfunction from intraoperative rewarming would extend into the first postoperative day; however, most patients in both groups failed to return to their preoperative baseline for CBF autoregulation. End-tidal CO2 and in-line arterial CO2 levels were monitored continuously to maintain normocarbia during mechanical lung ventilation during anesthesia and during CPB, respectively. We did not, however, measure CO2 levels after tracheal extubation postoperatively. Mild hypercarbia on postoperative day 1 in spontaneously ventilating patients might lead to impaired autoregulation. Regardless, while these differences between groups might explain worse autoregulation in the CABG patients, it does not detract from the principle finding that CBF autoregulation is preserved in the LVAD patients. Our autoregulation measurements were from the superficial frontal lobe of the brain and therefore represent regional, not global, cerebral autoregulation. It is possible that areas of the brain with cerebral vascular disease might have impaired autoregulation despite normal findings in the frontal lobe.
Finally, using the cutoff of Mx ≥ 0.4 or COx ≥ 0.3 to indicate abnormal autoregulation is admittedly arbitrary. The exact Mx or COx indicating impaired autoregulation is not clear but is likely between 0.3 and 0.5.8,9,13,19 Data from patients with traumatic injury, in fact, revealed that Mx ≥ 0.3 had the highest sensitivity for predicting mortality and poor outcomes.19 The COx cutoff used to denote abnormal autoregulation is based on data combined from multiple experiments in piglets that showed that a COx of ≥0.3 has a sensitivity of 89%, specificity 64%, and likelihood ratio 2.5 for detecting the lower limit of autoregulation.32 Impaired autoregulation denotes a condition in which CBF is blood pressure passive. Nonetheless, cerebral vasoreactivity that mediates autoregulation is not totally absent even when blood pressure is below the autoregulation threshold.33 The consequences of CBF that is pressure dependent ultimately depend on a myriad of factors centered on the adequacy of cerebral blood oxygen supply versus demand, including the presence of cerebral vascular disease.
CONCLUSION
These data suggest that CBF autoregulation is preserved during and immediately after surgery in patients undergoing LVAD insertion. Blood pressure across the autoregulatory range would not likely increase the risk for cerebral ischemia with low blood pressure or cerebral hyperemia at high pressures.
Acknowledgements
Funded in part by a grant from the National Institutes of Health (R01 HL092259-01) to Dr. Hogue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fang J. Rise of the machines—left ventricular assist devices as permanent therapy for advanced heart failure. N Engl J Med. 2009;361:2282–2285. doi: 10.1056/NEJMe0910394. [DOI] [PubMed] [Google Scholar]
- 2.Joshi B, Ono M, Brown C, et al. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth Analg. 2012;114:503–510. doi: 10.1213/ANE.0b013e31823d292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lietz K, Brown K, Ali S, et al. The role of cerebral hyperperfusion in postoperative neurologic dysfunction after left ventricular assist device implantation for end-stage heart failure. J Thorac Cardiovasc Surg. 2009;137:1012–1019. doi: 10.1016/j.jtcvs.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Pagani F, Miller L, Russell S, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 5.Rogers J, Aaronson K, Boyle A, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 6.Slaughter M, Rogers J, Milano C, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 7.van Mook W, Rennenberg R, Schurink G, et al. Cerebral hyperperfusion syndrome. Lancet Neurol. 2005;4:877–888. doi: 10.1016/S1474-4422(05)70251-9. [DOI] [PubMed] [Google Scholar]
- 8.Czonsnyka M, Brady K, Reinhard M, et al. Monitoring of cerebrovascular autoregulation: Facts, Myths, and Missing Links. Neurocrit Care. 2009;10:373–386. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- 9.Joshi B, Brady K, Lee J, et al. Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg. 2010;110:321–328. doi: 10.1213/ANE.0b013e3181c6fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ono M, Joshi B, Brady K, et al. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth. 2012 Jun 1; doi: 10.1093/bja/aes148. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellapart J, Chan G, Tzeng Y, et al. The effect of Ventricular Assist Devices on cerebral autoregulation: A preliminary study. BMC Anesthesiol. 2011;22:4. doi: 10.1186/1471-2253-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brady K, Joshi B, Zweifel C, et al. Real time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41:1951–1956. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brady K, Lee J, Kibler K, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38:2818–2825. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zweifel C, Castellani G, Czosnyka M, et al. Continuous assessment of cerebral autoregulation with near-Infrared spectroscopy in adults after subarachnoid hemorrhage. Stroke. 2010;41:1963–1968. doi: 10.1161/STROKEAHA.109.577320. [DOI] [PubMed] [Google Scholar]
- 15.STS . Adult Cardiac Database Data Specifications. The Society of Thoracic Surgeons; 2011. http://www.sts.org/sites/default/files/documents/word/STSAdultCVDataSpecificationsV2_73%20with%20correction.pdf. [Google Scholar]
- 16.Gottesman R, Grega M, Bailey M, et al. Delirium after coronary artery bypass graft surgery and late mortality. Annals of Neurology. 2010;67:338–344. doi: 10.1002/ana.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuitunen A, Vento A, Suojaranta-Ylinen R, et al. Acute renal failure after cardiac surgery: evaluation of the RIFLE classification. Ann Thorac Surg. 2006;81:542–546. doi: 10.1016/j.athoracsur.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 18.Diedler J, Zweifel C, Budohoski K, et al. The limitations of near-infrared spectroscopy to assess cerebrovascular reactivity: the role of slow frequency oscillations. Anesth Analg. 2011;113:849–857. doi: 10.1213/ANE.0b013e3182285dc0. [DOI] [PubMed] [Google Scholar]
- 19.Sorrentino E, Budohoski K, Kasprowicz M, et al. Critical thresholds for transcranial Doppler indices of cerebral autoregulation in traumatic brain injury. Neurocrit Care. 2011;14:188–193. doi: 10.1007/s12028-010-9492-5. [DOI] [PubMed] [Google Scholar]
- 20.Schell R, Kern F, Greeley W, et al. Cerebral blood flow and metabolism during cardiopulmonary bypass. Anesth Analg. 1993;76:849–865. doi: 10.1213/00000539-199304000-00029. [DOI] [PubMed] [Google Scholar]
- 21.Undar A, Eichstaedt H, Bigley J, et al. Effects of pulsatile and nonpulsatile perfusion on cerebral hemodynamics investigated with a new pediatric pump. J Thorac Cardiovasc Surg. 2002;124:413–416. doi: 10.1067/mtc.2002.125209. [DOI] [PubMed] [Google Scholar]
- 22.Hogue C, Jr., Palin C, Arrowsmith J. Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg. 2006;103:21–37. doi: 10.1213/01.ANE.0000220035.82989.79. [DOI] [PubMed] [Google Scholar]
- 23.Grubhofer G, Mares P, Rajek A, et al. Pulsatility does not change cerebral oxygenation during cardiopulmonary bypass. Acta Anaesthesiol Scand. 2000;44:586–591. doi: 10.1034/j.1399-6576.2000.00517.x. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto K, Onoguchi K, Takakura H, et al. Beneficial effect of balloon-induced pulsatility on brain oxygenation in hypothermic cardiopulmonary bypass. J Cardiovasc Surg (Torino) 2001;42:587–593. [PubMed] [Google Scholar]
- 25.Henze T, Stephan H, Sonntag H. Cerebral dysfunction following extracorporeal circulation for aortocoronary bypass surgery: no differences in neuropsychological outcome after pulsatile versus nonpulsatile flow. Thorac Cardiovasc Surg. 1990;38:65–68. doi: 10.1055/s-2007-1013995. [DOI] [PubMed] [Google Scholar]
- 26.Murkin JM, Martzke JS, Buchan AM, et al. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. I. Mortality and cardiovascular morbidity. J Thorac Cardiovasc Surg. 1995;110:340–348. doi: 10.1016/S0022-5223(95)70229-6. [DOI] [PubMed] [Google Scholar]
- 27.Giller C. The frequency-dependent behavior of cerebral autoregulation. Neurosurgery. 1990;27:362–368. doi: 10.1097/00006123-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Claassen J, Levine B, Zhang R. Dynamic cerebral autoregulation during repeated squat-stand maneuvers. J Appl Physiol. 2009;106:153–160. doi: 10.1152/japplphysiol.90822.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navarro J, Lao A, Sharma V, et al. The accuracy of transcranial Doppler in the diagnosis of middle cerebral artery stenosis. Cerebrovasc Dis. 2007;23:325–330. doi: 10.1159/000099130. [DOI] [PubMed] [Google Scholar]
- 30.Tsivgoulis G, Sharma V, Lao A, et al. Validation of transcranial Doppler with computed tomography angiography in acute cerebral ischemia. Stroke. 2007;38:1245–1249. doi: 10.1161/01.STR.0000259712.64772.85. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Brady K, Mytar J, et al. Cerebral blood flow and cerebrovascular autoregulation in a swine model of pediatric cardiac arrest and hypothermia. Crit Care Med. 2011;39:2337–2345. doi: 10.1097/CCM.0b013e318223b910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brady K, Mytar J, Kibler K, et al. Noninvasive autoregulation monitoring with and without intracranial pressure in the naive piglet brain. Anesth Analg. 2010;111:191–195. doi: 10.1213/ANE.0b013e3181e054ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Kibler K, Benni P, et al. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke. 2009;40:1820–1826. doi: 10.1161/STROKEAHA.108.536094. [DOI] [PubMed] [Google Scholar]