Figure 1.
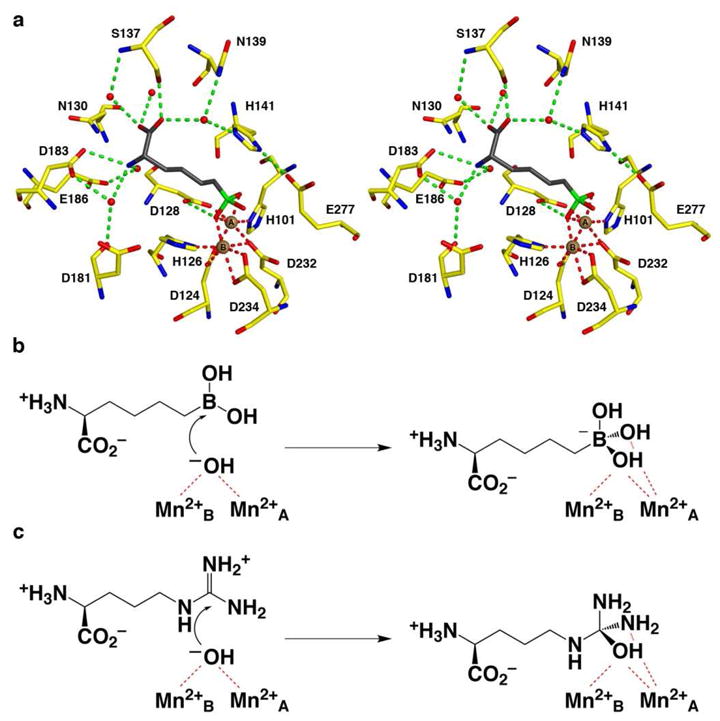
(a) Binding of the reactive substrate analogue inhibitor ABH in the active site of Mn2+2-human arginase I (PDB entry 2AEB), which mimics the binding of the tetrahedral intermediate and its flanking transition states in catalysis. Atoms are color-coded as follows: C = yellow, N = blue, O = red, B = green, Mn2+ = brown spheres, solvent = red spheres. (b) The nucleophilic metal-bridging hydroxide ion of Mn2+2-human arginase I attacks the sp2-hybridized boron atom in the trigonal planar boronic acid moiety of ABH to form a stable tetrahedral boronate anion. (c) The nucleophilic metal-bridging hydroxide ion of Mn2+2- human arginase I attacks the sp2-hybridized carbon atom in the trigonal planar guanidinium group of substrate L-arginine to form a metastable tetrahedral intermediate during catalysis.
